ABSTRACT: Atrial fibrillation (AFib), the most common type of cardiac arrhythmia, is associated with a diverse clinical presentation that can vary from patient to patient and imposes significant health and economic burdens on the patient and healthcare system. Individuals with AFib are five times more likely to have a stroke and other complications. While some patients are asymptomatic, common signs and symptoms include heart palpitations, irregular heartbeat, tachycardia, fatigue, and dizziness. In general, treatment goals are to control heart rate and rhythm, prevent the incidence of thromboembolism/stroke, and identify and address underlying risk factors. Treatment for AFib should be based on shared decision-making between the patient and clinician and often entails pharmacologic and nonpharmacologic measures tailored to patient needs. Pharmacists are well poised to identify patients at risk for and/or exhibiting signs of AFib and can be instrumental in making clinical recommendations regarding treatment, management, and prevention using a patient-centered approach to care. In November 2023, the American College of Cardiology, the American Heart Association, the American College of Chest Physicians, and the Heart Rhythm Society issued an updated guideline for preventing and optimally managing AFib.
Atrial fibrillation, commonly referred to as AFib, is defined as “a supraventricular tachyarrhythmia that is characterized by a diffuse and disorganized atrial electrical activity that replaces normal sinus node function and results in ineffective mechanical atrial contraction.”1 Left undiagnosed and untreated, AFib is associated with enormous health and economic burdens, considerable cardiac morbidity including the augmented risk of and/or exacerbation of heart failure (HF), increased healthcare resource utilization including office visits, emergency department (ED) visits, and hospitalizations, increased risk of thromboembolic events/stroke, cognitive dysfunction, dementia, and high rates of mortality.1-4 According to the U.S. Preventive Services Task Force (USPSTF), an estimated 20% of patients who experience a stroke/thromboembolic event due to AFib are first diagnosed with AFib at the time of receiving treatment for the stroke or not long after.5
As integral members of the healthcare team, it is imperative that pharmacists are knowledgeable about AFib and understand their roles as clinicians and patient educators in making clinical recommendations about possible therapies. Pharmacists can also be instrumental in educating patients about risk factors, recommended measures to reduce or prevent AFib-related complications, and the significance of adherence to selected therapy and routine follow-up with their primary healthcare provider.
Prevalence of AFib
AFib is the most commonly occurring type of cardiac arrhythmia and the principal cardiac cause of stroke/thromboembolic events. It is the most frequently treated arrhythmia in EDs and has been documented to account for over one-third of all arrhythmia-related hospitalizations.1-3 Research has established that the incidence of AFib increases with age, and its prevalence ranges from 0.2% among those aged younger than 55 years to 10% among individuals aged 85 years and older.5 Studies also reveal that in 2015, an estimated 11% (591,000) of the >5.6 million AFib cases in the United States were undiagnosed, highlighting the need to expand awareness about AFib and the significance of recognizing the signs, symptoms, and risk factors.6-8
The most recent statistics from the American Heart Association (AHA) indicate that AFib affects over 6 million Americans.2,4 The number of individuals impacted is projected to double by 2030.4 The CDC notes that, annually, an estimated 450,000 hospitalizations occur because of AFib.4 Another study indicated that as the aging population continues to grow, predictions suggest that the burden of AFib may increase by more than 60% by 2050.9 The rates of mortality from AFib have risen over the past 2 decades.9 The CDC also indicates that in 2019, AFib was documented on 183,321 death certificates and noted as the underlying cause of death in 26,535 of those deaths.4,9,10 A recent publication in Nature Reviews Cardiology indicated that over the past 3 decades, the worldwide incidence of AFib has increased considerably and affects an estimated 60 million people.11 Studies also indicate that in addition to the growing aging population, the incidence of AFib is also due to the increasing number of individuals with chronic cardiovascular disease and other risk factors, including obesity, uncontrolled diabetes, and a sedentary lifestyle, as well as increased recognition of AFib.12
Updated Guideline
In November 2023, the American College of Cardiology (ACC), the AHA, the American College of Chest Physicians (ACCP), and the Heart Rhythm Society (HRS) updated their guideline for the management and prevention of AFib, which the American College of Clinical Pharmacy endorsed. The 2023 ACC/AHA/ACCP/HRS Guideline for Diagnosis and Management of Atrial Fibrillation was published in the Journal of the American College of Cardiology (JACC) and Circulation. The updated recommendations provide clinicians with critical information to aid in the management and treatment of AFib and accentuate the value of a multifaceted and patient-centered approach to therapy, including a robust implementation of lifestyle modifications to reduce or lessen the health burdens associated with AFib. The guideline suggests early aggressive strategies for rhythm control, provides valuable information regarding the advancements in therapeutic interventions, and updates the guidance regarding the management of heart rate and rhythm control drugs, the use of anticoagulants, and recommendations to pause or discontinue therapies when warranted.13 Additionally, the guideline emphasizes a strategy for rhythm control that is comparable to what is recommended in the most recent AFib guideline from the European Society of Cardiology.13,14
Updated Stages of AFib
The updated guideline notes that the previous classification of AFib was established only on the duration of the arrhythmia, and the expert writing committee indicated that, while beneficial, it tended to highlight therapeutic interventions.13,15 The new proposed classification employs stages and acknowledges AFib as a disease continuum that entails various strategies at the different stages, including prevention, modification of lifestyle and risk factors, screening, and therapy.13,15
The updated guideline proposes a new classification system and stages of AFib, including:
Stage 1: At risk for AFib due to the presence of risk factors.
Stage 2: Pre-AFib, with evidence of structural or electrical findings predisposing to AFib.
Stage 3: AFib, including paroxysmal (3A), persistent (3B), long-standing persistent (3C), and successful AFib ablation (3D; patients may transition among different substages of AFib).
Stage 4: Permanent AFib (no further attempts at rhythm control after discussion between patient and healthcare provider).13,15
Risk Factors and Clinical Presentation
Literature indicates that AFib is associated with a 1.5-fold to twofold heightened risk of mortality, and data from studies suggest that the risk of mortality from AFib is typically greater among women when compared with men, with a documented 12% greater risk of mortality.13,15,16 AFib is also associated with approximately a fivefold augmented risk of stroke and HF.16 Examples of established risk factors for AFib include advancing age, sedentary lifestyle, obesity, uncontrolled hypertension, smoking and moderate-to-heavy alcohol use, uncontrolled diabetes, metabolic syndrome, undiagnosed and untreated obstructive sleep apnea, hyperthyroidism, being of European ancestry, ischemic cardiovascular disease (CVD), valvular CVD, HF, hyperlipidemia, chronic obstructive pulmonary disease, chronic kidney disease, and inflammatory diseases.6,11,13,15,16 The updated guideline recognizes lifestyle and risk factor modification as a “pillar of AFib management” to prevent the onset, progression, and adverse outcomes and also emphasizes risk factor management throughout the course of AFib, including management of obesity, weight loss, physical activity, smoking cessation, alcohol moderation, hypertension, and other comorbidities, as applicable.13,15 FIGURE 1 illustrates this guideline.
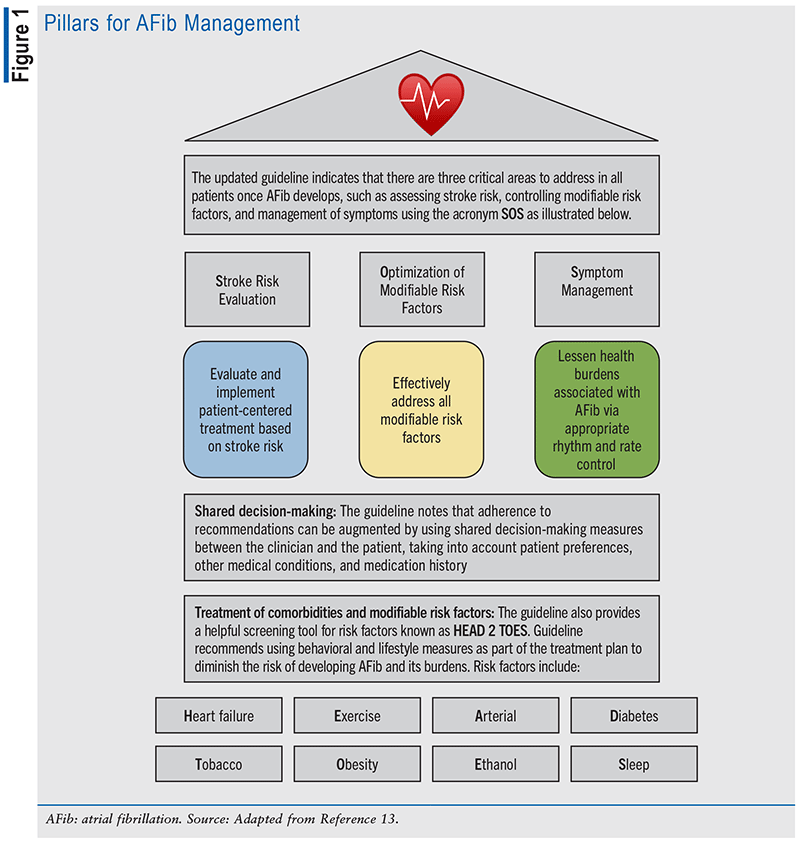
Clinical Presentation
The clinical presentation associated with AFib varies from patient to patient. The most common symptoms are heart palpitations, tachycardia, weakness, dizziness, fatigue, lightheadedness, mild dyspnea, increased urination, and reduced exercise capacity.16,17 According to the AHA, AFib often goes undiagnosed because many patients are asymptomatic, or the symptoms occur sporadically, with more than 33% of patients with AFib being asymptomatic. This leads to undetected and untreated cases, which add to the health and economic burdens, including high rates of morbidity and mortality and enormous direct and indirect costs as a result of the complications.18
Treatment, Management, and Prevention
In general, the goals of managing patients with AFib—using shared decision-making and a patient-centered approach—are to diminish the symptom burden, maintain sinus rhythm, and diminish or avert the risk of complications such as thromboembolic events/stroke.5 Once AFib is diagnosed, efficacious management of AFib warrants a multidisciplinary approach of pharmacologic and nonpharmacologic measures. These include acute management, identification and treatment of underlying and concomitant cardiovascular conditions and other risk factors, measures to thwart thromboembolic events/stroke, and managing the heart’s rate and rhythm control.13,15,19
Data from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study and comparable findings from the smaller, Rate Control Versus Electrical Cardioversion (RACE) trial have contributed to the advancement of consensus guidelines that endorse employing an initial rate-control strategy for the majority of asymptomatic patients with AFib.20
According to the USPSTF, to prevent or reduce the risk of a thrombotic event, the use of anticoagulants is employed in patients at elevated risk. These agents include the vitamin K antagonist (VKA) warfarin and direct oral anticoagulants (DOACs), such as those that act specifically on factor IIa (dabigatran) or factor Xa (i.e., rivaroxaban, apixaban, and edoxaban).5
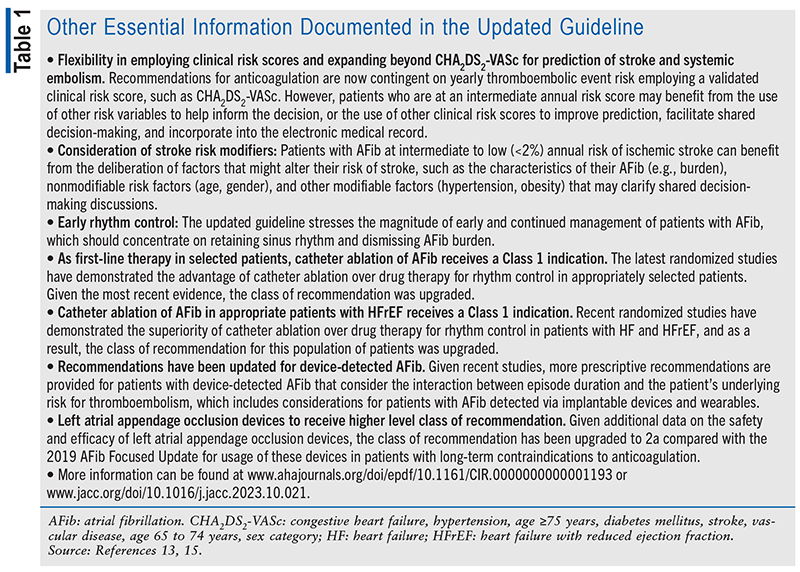
The updated guideline states, “Management, in accordance with guideline recommendations, is effective only when followed by both practitioners and patients. Adherence to recommendations can be enhanced by shared decision-making between clinicians and patients, with patient engagement in selecting interventions on the basis of individual values, preferences, and associated conditions and comorbidities.”13,15 The updated guideline indicates that the foundation of optimally managing AFib is identifying and treating the risk factors and implementing measures to decrease the probability of developing AFib and related complications, including treating comorbidities and risk factors.13,15 See TABLE 1 for additional information that was documented in the updated guideline.
Studies show that both rate control and rhythm control can enhance symptoms in patients with AFib.13,15 The selection between a rhythm-control or a rate-control strategy is contingent upon several factors, including patient age, the intensity to which symptoms interfere with the quality of life, and concerns about antiarrhythmic drug therapy or catheter ablation.16 The therapy selection should consider patient-specific characteristics, including other comorbidities and responses to previous therapies, if applicable.13,15
Rate Control
According to the guideline, nondihydropyridine calcium channel blockers (verapamil and diltiazem) slow conduction via the atrioventricular node, have negative inotropic and chronotropic effects, and are valuable in ventricular rate control in the absence of preexcitation.13,15 Calcium channel blockers also provide reasonable rate control and improve AFib-related symptoms compared with beta-blockers. Beta-blockers (i.e., metoprolol tartrate, metoprolol succinate, atenolol, carvedilol, esmolol, nadolol, and propranolol) also slow conduction through the atrioventricular node by blocking beta-1 receptors.13,15 The updated guideline indicates that digoxin is sometimes prescribed for patients with AFib, principally among patients with HF with reduced ejection fraction (HFrEF) due to its positive inotropic and vagotonic effects.13,15 The guideline also notes that amiodarone is prescribed as a rate-control therapy in patients who are intolerant of or deemed unresponsive to other agents, such as patients with congestive HF and/or those unable to tolerate diltiazem or metoprolol.13,15,20
Rhythm Control
Antiarrhythmic drug therapy aims to reduce the duration and frequency of AFib, decreasing symptom burden and enhancing the patient’s health-related quality of life and productivity.20 Examples of these agents include sodium channel blockers (flecainide propafenone and quinidine) and potassium channel blockers (amiodarone, sotalol, dofetilide, and dronedarone).13,15
According to the updated guideline, antiarrhythmic drugs are practical therapies for long-term maintenance of sinus rhythm for patients with AFib who are not candidates for or decline catheter ablation or who favor antiarrhythmic treatment, with the following recommendations:
• Flecainide and propafenone are selections for the maintenance of sinus rhythm in patients with no previous history of myocardial infarction or significant structural CVD.
• Dronedarone is an option for the maintenance of sinus rhythm in patients without recent decompensated HF or severe left-ventricle dysfunction.
• Dofetilide and sotalol are effective for maintaining sinus rhythm but are linked with torsades de pointes and require QT interval (the measurement of time from the start of the Q wave to the end of the T wave made on an ECG) monitoring.
• Sotalol is best avoided in patients with HFrEF because most patients are already prescribed a beta-blocker, and adding an additional beta-blocker is not likely to be well tolerated.13,15
Antithrombic Therapy
The USPSTF recommendations issued in 2022 note that to diminish the risk of stroke, anticoagulants such as warfarin (a VKA) and target-specific anticoagulants, also known as DOACs, are prescribed based on patient need.5 Most patients with AFib should receive a long-term oral anticoagulant (OAC) to decrease the risk of ischemic stroke and other embolic events, and the literature also indicates that in the majority of patients, the benefit from anticoagulation outweighs the associated increase in the risk of bleeding.16 The updated guideline indicates that DOACs were established to address the shortcomings of warfarin and are presently recommended as the first-line therapy over warfarin in patients with AFib (except for moderate-to-severe mitral stenosis or mechanical heart valve recipients).13,15 The guideline also indicates that for the prevention of stroke or systemic embolism in patients with AFib, except for moderate-to-severe mitral stenosis or mechanical heart valve, data from all four pivotal clinical trials comparing individual DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) with warfarin exhibited superiority or noninferiority to warfarin.13,15
To ensure the optimal selection of an OAC in patients with AFib, multiple factors should be considered, including efficacy, safety, other comorbidities, insurance coverage, renal/hepatic function, potential drug/drug interactions, screening for contraindications, designing a treatment plan that will improve medication adherence, and patient preferences.13,15
Recent Clinical Data and News
A recent publication in JACC: Clinical Electrophysiology revealed that poor sleep was associated with a direct increased risk for self-reported episodes of AFib, and increasingly worse sleep was linked with longer episodes of AFib the next day. The authors concluded that their data imply that quality of sleep may be a modifiable trigger significant to the near-term risk of an isolated AFib episode.21
According to a presentation at the AHA Scientific Sessions 2023, data from the AZALEA-TIMI 71 trial showed that an investigational anticlotting agent, abelacimab, meaningfully lowered bleeding by more than 60% among patients with AFib. Researchers also revealed that the trial was halted early in September 2023 due to the “overwhelming reduction” in bleeding with abelacimab.21 Data revealed that abelacimab was well tolerated, with comparable rates of adverse events compared with rivaroxaban.22
Another study published in the Journal of the American Heart Association revealed that sleep-related hypoxia in patients with sleep apnea was correlated with incident AFib.22 The researchers discovered that for every 10%-point decline in average oxygen saturation rate, the risk of AFib was heightened by as much as 30%. As a result of their findings, the researchers recommended routine sleep apnea screening and treating it to reduce the risk of developing AFib.23
A recent study published in Circulation: Cardiovascular Quality and Outcomes revealed that more than one in 10 patients hospitalized for AFib are discharged with a prescription for an off-label DOAC dose, with considerable differences across hospitals. Based on their findings, the researchers concluded that over time, while the percentage of patients receiving the recommended dosing has notably advanced, there are still opportunities to enhance DOAC dosing among eligible patients.24
The Role of the Pharmacist
Due to their drug expertise, pharmacists can be instrumental in the multidisciplinary care of patients with AFib by identifying patients with risk factors and/or those exhibiting signs associated with AFib and encouraging these patients to seek further medical evaluation from their primary healthcare provider. Pharmacists can also counsel patients about the available treatments and advise them on the proper administration and possible adverse effects associated with these agents, as well as screen for potential drug-drug interactions and contraindications, monitor patient response, and make clinical recommendations when warranted to optimize patient adherence and clinical outcomes.
The updated guideline indicates that screening by pharmacists for potential drug-drug interactions in patients with AFib provides clinical benefits.13,15
Conclusion
Early detection and clinical intervention tailored to patient needs can significantly decrease and/or reduce the complications associated with AFib, improve clinical outcomes in patients, and enable patients to live normal and active lives. Empowering patients with knowledge about AFib and therapies can be instrumental in decreasing and addressing the symptom burdens and improving patients’ overall health-related quality of life. Pharmacists can also direct patients to valuable resources such as:
StopAfib.org: www.stopafib.org
AFib resources: www.heart.org/en/health-topics/atrial-fibrillation/afib-resources-for-patients--professionals
Living with AFib: www.heartrhythmalliance.org/afa/us
Atrial fibrillation: www.heart.org/en/health-topics/atrial-fibrillation
REFERENCES
1. Nayak S, Natarajan B, Pai RG. Etiology, pathology, and classification of atrial fibrillation. Int J Angiol. 2020;29(2):65-71.
2. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528.
3. Nesheiwat Z, Goyal A, Jagtap M. Atrial fibrillation. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-.
4. American Heart Association. Early rhythm control, lifestyle modification and more tailored stroke risk assessment are top goals in managing atrial fibrillation. November 30, 2023. www.newsroom.heart.org/news/early-rhythm-control-lifestyle-modification-and-more-tailored-stroke-risk-assessment-are-top-goals-in-managing-atrial-fibrillation. Accessed February 4, 2024.
5. US Preventive Services Task Force. Screening for atrial fibrillation: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(4):360-367.
6. Andrade JG, Aguilar M, Atzema C, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36:1847-1948.
7. Turakhia MP, Guo JD, Keshishian A, et al. Contemporary prevalence estimates of undiagnosed and diagnosed atrial fibrillation in the United States. Clin Cardiol. 2023;46(5):484-493.
8. CDC. Atrial fibrillation. October 2022. www.cdc.gov/heartdisease/atrial_fibrillation.htm. Accessed December 1, 2023.
9. Trohman RG, Huang HD, Sharma PS. Atrial fibrillation: primary prevention, secondary prevention, and prevention of thromboembolic complications: part 2. Front Cardiovasc Med. 2023;9:1060096.
10. CDC. About multiple cause of death, 1999–2019. CDC WONDER Online Database website. Atlanta, GA: CDC; 2019. https://wonder.cdc.gov/mcd-icd10.html. Accessed January 8, 2024.
11. Elliott AD, Middeldorp ME, Van Gelder IC, et al. Epidemiology and modifiable risk factors for atrial fibrillation. Nat Rev Cardiol. 2023;20:404-417.
12. Brandes A, Smit MD, Nguyen BO, et al. Risk factor management in atrial fibrillation. Arrhythm Electrophysiol Rev. 2018;7(2):118-127.
13. Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2024;83(1):109-279
14. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2021;42:373-498.
15. Joglar JA, Chung MK, Anmbraster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156.
16. Streur M. Atrial fibrillation symptom perception. J Nurse Pract. 2019;15(1):60-64.
17. Kumar K. Atrial fibrillation: overview and management of new-onset atrial fibrillation. UpToDate. www.uptodate.com/contents/atrial-fibrillation-overview-and-management-of-new-onset-atrial-fibrillation. Accessed January 13, 2024.
18. Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127(1):4-20.
19. Lundqvist CB, Pürerfellner H, White A, Schilling R. Redefining the standard for atrial fibrillation: a patient-centric report. Eur Cardiol. 2021;16:(Suppl 1).
20. Rosenthal L. Atrial fibrillation treatment & management. Medscape. November 18, 2019. https://emedicine.medscape.com/article/151066-treatment?&icd=login_success_email_match_fpf#d10. Accessed January 5, 2024.
21. Wong CX, Modrow MF, Sigona K, et al. Preceding night sleep quality and atrial fibrillation episodes in the I-STOP-AFIB randomized trial. JACC Clin Electrophysiol. 2023:S2405-500X(23)00729-6.
22. American Heart Association. New anti-clotting medication reduces bleeding among people with atrial fibrillation. November 12, 2023. www.newsroom.heart.org/news/new-anti-clotting-medication-reduces-bleeding-among-people-with-atrial-fibrillation. Accessed December 1, 2023.
23. Heinzinger CM, Thompson NR, Millinovich A, et al. Sleep-disordered breathing, hypoxia, and pulmonary physiologic influences in atrial fibrillation. J Am Heart Assoc. 2023;12:e031462.
24. Sandhu A, Kaltenbach LA, Chiswell K, et al. Off-label dosing of direct oral anticoagulants among inpatients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2023;16(12):e010062.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






