US Pharm. 2023;48(12):14.
According to the National Cancer Institute, colorectal cancer (CRC) is the fourth most commonly diagnosed cancer and the second leading cause of cancer-related deaths in the United States. The median age at CRC diagnosis is 66 years, and the median age of death from CRC is 72 years. It was estimated that 153,020 adults would be diagnosed with CRC in 2023, and 52,550 would die as a result of CRC. Effective, reliable screening has led to a substantial (>30%) decrease in CRC incidence and mortality among adults aged >50 years over the past 15 years.
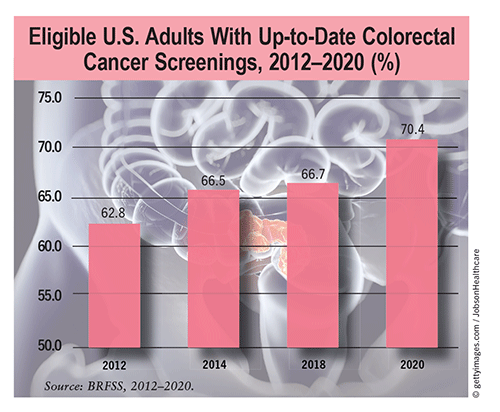
Status: A cross-sectional study of Behavioral Risk Factor Surveillance System (BRFSS) data discovered a significant increase between 2012 and 2020 in the percentage of up-to-date CRC screenings in adults aged 50 to 75 years without a history of CRC (from 62.8% to 70.4%). These screening rates were based on the 2008 U.S. Preventive Services Task Force recommendations, which were subsequently updated in 2021 to include individuals at average risk who were aged >45 years (rather than >50 years). This change in the recommended age was based on a rise in the incidences of colon cancer and rectal cancer among individuals aged <50 years over the past several decades (from 1% to 2.4% and from 2.3% to 3.2%, respectively).
Disparities: CRC screening trends over the past decade have been found to relate to certain sociodemographic characteristics and U.S. Census Bureau geographic divisions. The study of BRFSS data for adults aged 50 to 75 years without a history of CRC revealed that up-to-date CRC screening rates increased significantly between 2014 and 2020 among non-Hispanic whites (68.5% to 74.5%), non-Hispanic blacks (68% to 74.6%), and Hispanics (51.5% to 62.8%). However, all increases remained below the National Colorectal Cancer Roundtable target of 80%. Increases in up-to-date CRC screenings among eligible individuals were also noted in all Census Bureau geographic divisions except the Pacific Division, which had a nonsignificant decrease of 2.2% from 2014 to 2020. The New England Division reported the greatest overall proportion of up-to-date CRC screenings, consistently >70% for the same time period.
Tests: Screening tests can detect CRC prior to the onset of symptoms, potentially leading to more timely treatment and reductions in CRC-related deaths. Numerous approaches are used to screen for CRC, including stool-based tests (e.g., fecal occult blood test [FOBT], stool DNA testing, fecal immunochemical testing) and optical/visualization tests (e.g., sigmoidoscopy, colonoscopy, virtual colonoscopy [CT colonography]). As of 2020, colonoscopy was the most-used CRC screening test (64.5%) in the U.S., followed by FOBT (12.6%), stool DNA testing (5.8%), sigmoidoscopy (3.8%), and virtual colonoscopy (2.7%).
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





