US Pharm. 2024;49(3):34-37.
ABSTRACT: At present, diffuse large B-cell lymphoma (DLBCL) is the most common lymphoid malignancy in Western countries. The initial response rate to first-line therapy is about 60%, and drug development and investigation are targeting the 30% to 40% relapse rate, 10% refractory rate, and regimens for frail and elderly patients. In the past few years, new drug classes for DLBCL treatment have been approved. In 2023, the FDA approved the first bispecific T-cell engager agents, epcoritamab-bysp and glofitamab-gxbm. These two agents constitute new third-line options for patients with relapsed or refractory DLBCL. Pharmacists are an integral part of oncology management because they can assist with regimen selection, monitoring, patient education, and identification of clinical trials.
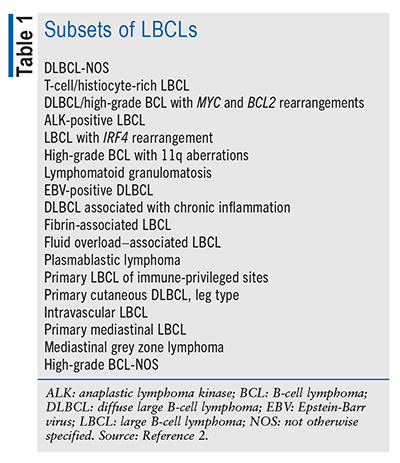
Lymphoma is an overarching term for malignancies that involve the lymphocytes and lymphatic system, including the lymph nodes, spleen, thymus, and bone marrow.1 The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours categorizes lymphoid tumors hierarchically, with the initial stratification based on cell type (B cell, T cell, or natural killer cell).2 Lymphomas are more commonly categorized as either non-Hodgkin’s or Hodgkin’s lymphoma.1 Large B-cell lymphomas (LBCLs) are a class of mature B-cell neoplasms comprising 18 different subsets (TABLE 1); the most common form is diffuse large B-cell lymphoma, not otherwise specified (DLBCL-NOS), which is the focus of this article.2 Accounting for around 30% of all non-Hodgkin’s lymphoma diagnoses in Western countries, DLBCL is the most common lymphoid malignancy.3 In the United States, non-Hodgkin’s lymphoma is the 8th most common type of cancer diagnosed.4 The current rate of new cases of DLBCL is 5.5 per 100,000 men and women, with a death rate of 1.7 per 100,000 individuals.5
Based on National Cancer Institute Surveillance, Epidemiology, and End Results Program data for the U.S. from 2016 to 2020, DLBCL was more common in men than in women (6.6 per 100,000 cases vs. 4.5 per 100,000, respectively).5 Hispanic individuals had a higher-than-average incidence, at 7.2 per 100,000 cases for men and 5.4 per 100,000 for women. Older age is associated with DLBCL, as the median age at diagnosis is 66 years. Although most DLBCL cases are primary (de novo) in nature and have no known cause, a small number are attributable to the progression or transformation of less aggressive lymphomas.3 Studies have demonstrated an increased risk of primary disease in patients with family history and genetic susceptibility; viruses such as Epstein-Barr, HIV, or hepatitis C; solid-organ transplant; B cell–activating autoimmune disorders; immunodeficiency; increased BMI in young adulthood; occupational exposures (pesticides and fertilizers); and ionizing radiation.3,6,7
Diagnosis
Most cases of DLBCL are devoid of systemic symptoms, with patients presenting with painless, increasing adenopathy at a single or extranodal site.1,3 Extranodal sites may include the kidneys, adrenal gland, brain, other soft tissue, and bones.8 Systemic symptoms, or B symptoms, are caused by cytokine release and may include fever, unexplained weight loss of >10% of body weight in 6 months, and night sweats.1 The presence of B symptoms is associated with advanced disease.6 If enlarged, suspicious-appearing lymph nodes are identified on physical examination and/or radiographic imaging, an excisional biopsy should be performed.
DLBCL morphology is marked by a large, transformed B cell with a nuclear diameter measuring more than twice that of a normal lymphocyte. Immunohistochemistry, flow cytometry, fluorescence in situ hybridization (FISH), and molecular testing also are conducted to provide an accurate lymphoma diagnosis, determine prognosis, and guide treatment selection.6 Immunohistochemistry identifies the expression of pan-B cell antigens (cluster of differentiation [CD] 19, CD20, CD22, CD79a, and CD45) and surface immunoglobulin +/− and cytoplasmic immunoglobulin −/+.3 Additional markers may be present depending on variances in plasmacellular differentiation and morphology. Gene expression profiling can delineate two distinct molecular subtypes of DLBCL, the germinal center B-cell–like subtype and the activated B-cell–like subtype; however, 10% to 15% of DLBCL cases are considered unclassifiable.6 FISH may be used to determine recurrent genetic rearrangements, such as MYC, BCL2, or BCL6 rearrangements. At present, therapy is not guided by subtypes or genetic rearrangements, but there is evidence of a correlation between differences in outcomes and response to treatment.
Staging and Prognostic Factors
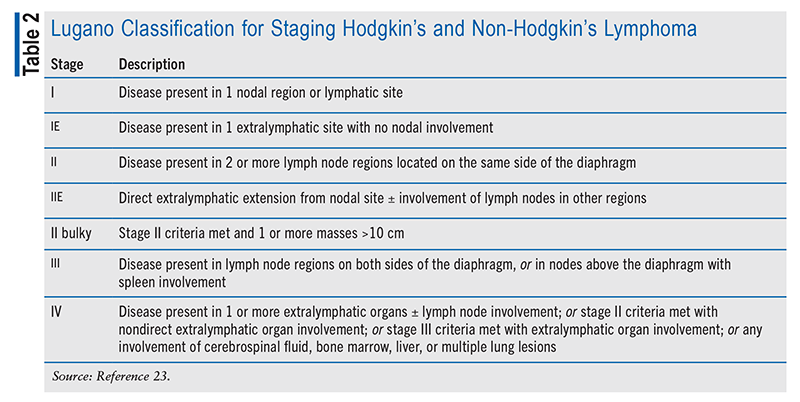
Prior to the availability of fluorodeoxyglucose positron emission tomography with CT (PET-CT), the staging of DLBCL was based solely on the Ann Arbor staging system.1,3,6 The Ann Arbor system considers the number of sites of involvement, the occurrence of B symptoms, and the presence of extranodal disease. The Lugano classification incorporates PET-CT results to determine the disease stage (TABLE 2). Clinicians use the International Prognostic Index (IPI) to determine prognosis, assess patients’ eligibility for clinical trials, and guide treatment regimens.3 The IPI categorizes risk as low, low-intermediate, high-intermediate, or high based on age, disease stage, lactate dehydrogenase level, Eastern Cooperative Oncology Group performance score, and extranodal disease.
Treatment
Approved in 1997, rituximab was the first in a series of new chemotherapeutic agents for lymphoma that have changed the landscape of DLBCL treatment.9,10 Standard care for DLBCL has a relapse rate of approximately 30% to 40% and a refractory rate of 10%.11 If DLBCL is left untreated at this stage, the prognosis is poor, with a life expectancy of only about 3 to 4 months. For this reason, the focus of new agents for treating DLBCL has been on patients with relapsed or refractory disease.
First-Line Therapy: Given its success rate of approximately 60%, RCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) has been a mainstay of therapy since clinical trials began in the early to mid-1990s.9 The current National Comprehensive Cancer Network guideline lists the RCHOP regimen as the sole first-line chemotherapy option for patients with stage I or II disease, with cycle length and incorporation of radiation therapy based on the presence of bulky disease.12 In patients with stage IIE, III, or IV disease, RCHOP is still preferred, as is Pola-R-CHP (polatuzumab vedotin-piiq, rituximab, cyclophosphamide, doxorubicin, prednisone); dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin) + rituximab is noted to be used only as recommended. Although RCHOP is not recommended in patients who have poor left ventricular function, are very frail, or are aged >80 years with comorbidities, alternative regimens remain similar, and rituximab, cyclophosphamide, and prednisone are always incorporated.
The use of polatuxumab vedotin-piiq (Polivy) in conjunction with a rituximab product, cyclophosphamide, doxorubicin, and prednisone as an alternative first-line therapy received FDA approval in April 2023 based on the POLARIX trial.13 Polatuxumab vedotin-piiq was initially approved by the FDA in 2019, in conjunction with bendamustine and a rituximab product, for use in patients with relapsed or refractory DLBCL-NOS after at least two prior therapies.14 This agent is a CD79b-directed antibody and microtubule inhibitor conjugate that acts on B cells by inhibiting cell division and inducing apoptosis.
Second-Line Therapy: Second-line therapy is employed in patients who have relapsed or refractory disease following first-line therapy.12 After a patient initiated on first-line therapy has received three cycles, an interim restaging PET-CT is conducted. Response is stratified based on the Lugano classification criteria, with three outcomes possible: complete response (CR), partial response, or progressive disease. In patients who demonstrate response, the course of therapy may be completed as planned, extended, or dose-adjusted. Upon attainment of CR and completion of treatment, patients transition to follow-up, wherein they are monitored with laboratory testing every 3 to 6 months for 5 years and yearly thereafter, as well as biannual or annual CT scans for the first 2 years. Disease that recurs following CR is considered to be relapsed; when a patient does not achieve CR or the disease is progressive, it is considered refractory.
Therapeutic options vary based on the timing of relapse (within or after 12 months) and when disease was deemed refractory to treatment.12 For some patients, second-line therapy may include stem-cell transplantation, participation in a clinical trial, palliative radiation, or supportive care. The number of second-line therapies is extensive, with anti-CD19 chimeric antigen receptor (CAR) T-cell therapy being a new option for LBCL treatment. Two agents in that class, axicabtagene ciloleucel (Yescarta) and lisocabtagene maraleucel (Breyanzi), may be used for second- and third-line therapy. Anti-CD19 CAR T-cell agents are engineered to harness the body’s natural immune function to induce cytotoxic killing of B cells.15 To prepare the anti-CD19 CAR T-cell agent, the patient’s T cells are harvested; genetically modified to express a CAR against CD19; and then replicated and expanded, washed, and placed into suspension for reinfusion. After administration, binding of CAR to diseased and normal B cells induces activation and proliferation of CAR T cells, release of proinflammatory cytokines, and cytotoxic killing of target cells. Risk Evaluation and Mitigation Strategy (REMS) programs are required for these agents because of the high risk of cytokine release syndrome and neurologic events, which are potentially life-threatening and may have a delayed onset.16 Secondary T-cell malignancies have occurred after CAR T-cell therapy; the FDA is investigating this risk, and healthcare providers should report events to MedWatch (www.fda.gov/medwatch).17
Third-Line Therapy: At present, third-line and subsequent therapies are categorized as either T-cell mediated or non–T-cell mediated.12 T-cell mediated therapy is subdivided into anti-CD19 CAR T-cell agents and bispecific T-cell engager agents (discussed in more detail below). The anti-CD19 CAR T-cell agent tisagenlecleucel is recommended only as a third-line option. Non–T-cell mediated therapies include loncastuximab tesirine-lpyl (Zynlonta) and selinexor (Xpovio).
In 2023, the FDA approved the first bispecific T-cell engager agents—epcoritamab-bysp and glofitamab-gxbm—for patients with different forms of LBCL.18 Epcoritamab-bysp (Epkinly) received approval in May 2023 for patients with relapsed or refractory DLBCL-NOS and high-grade BCL after two or more lines of systemic therapy. Glofitamab-gxbm (Columvi) was approved in June 2023 to treat DLBCL-NOS or LBCL arising from follicular lymphoma after two or more lines of systemic therapy. Bispecific T-cell engager therapy works by simultaneously binding to antigens on tumor cells and CD3 subunits on T cells.19 The dual binding enables T-cell recruitment to the diseased cell as well as T-cell activation and cytotoxic killing. Although both agents carry a black box warning for cytokine release syndrome and epcoritamab-bysp has a black box warning for immune effector cell–associated neurotoxicity syndrome, there are no associated REMS programs for these agents.20,21 Other adverse effects include fatigue, musculoskeletal pain, gastrointestinal issues, and cytopenia.
Emerging Therapies: Similar to other recently approved agents, several clinical trials for DLBCL involve agents that have already been approved for other forms of lymphoma or were originally approved in conjunction with different agents as a specified treatment regimen.22 Other bispecific T-cell engager agents (zanubrutinib [Brukinsa], acalabrutinib [Calquence], mosunetuzumab [Lunsumio]) may gain FDA approval for treatment-naïve elderly patients with DLBCL. Research to create more effective or specific agents continues. Phase I and II trials are currently ongoing for maplirpacept (PFs-07901801) and zandelisib (ME-401) in DLSCL, and previous studies in other forms of lymphoma seem promising. Novel drug classes being investigated for DLBCL include TIGIT (T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domains) inhibitors, anti–programmed death receptor 1 antibodies, tetra-specific antibodies, embryonic ectoderm development polycomb repressive complex 2 inhibitors, anti-CD74 antibodies, dual phosphoinositide 3-kinase/histone deacetylase inhibitors, and interleukin-15 receptor agonists.
Conclusion
The landscape of cancer therapy has changed markedly in the past 30 years and will keep evolving as new drug targets and formulations are developed. Given regimens’ complexity, the array of adverse effects, and varying monitoring parameters, collaboration of a team of healthcare professionals is essential to ensure optimal patient care. Pharmacists are a key part of oncology management because they can assist with regimen selection, monitoring, patient education, and identification of clinical trials. Providing current information to the healthcare team is another service pharmacists can perform as development of drugs and regimens continues.
REFERENCES
1. Lewis WD, Lilly S, Jones KL. Lymphoma: diagnosis and treatment. Am Fam Physician. 2020;101(1):34-41.
2. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720-1748.
3. Martelli M, Ferreri AJM, Agostinelli C, et al. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013;87(2):146-171.
4. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: non-Hodgkin lymphoma. https://seer.cancer.gov/statfacts/html/nhl.html. Accessed June 30, 2023.
5. National Cancer Institute Surveillance, Epidemiology and End Results Program. Cancer stat facts: NHL—diffuse large B-cell lymphoma (DLBCL). https://seer.cancer.gov/statfacts/html/dlbcl.html. Accessed June 30, 2023.
6. Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021;384(9):842-858.
7. Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v116-v125.
8. Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94(5):604-616.
9. Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22(5):941-952.
10. Grillo-López AJ, White CA, Dallaire BK, et al. Rituximab: the first monoclonal antibody approved for the treatment of lymphoma. Curr Pharm Biotechnol. 2000;1(1):1-9.
11. Raut LS, Chakrabarti PP. Management of relapsed-refractory diffuse large B cell lymphoma. South Asian J Cancer. 2014;3(1):66-70.
12. Zelenetz AD, Gordon LI, Abramson JS, et al. NCCN Guidelines® insights: B-cell lymphomas, version 6.2023. J Natl Compr Canc Netw. 2023;21(11):1118-1131.
13. FDA. FDA D.I.S.C.O. burst edition: FDA approval of Polivy (polatuzumab vedotin-piiq) for previously untreated diffuse large B-cell lymphoma, not otherwise specified, and high-grade B-cell lymphoma. www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-polivy-polatuzumab-vedotin-piiq-previously-untreated-diffuse. Accessed January 9, 2024.
14. Polivy (polatuzumab vedotin-piiq) product information. South San Francisco, CA: Genentech, Inc; April 2023.
15. Abramson JS. Anti-CD19 CAR T-cell therapy for B-cell non-Hodgkin lymphoma. Transfus Med Rev. 2020;34(1):29-33.
16. McDermott K, Spendley L. Anti-CD19 CAR T-cell therapy for adult patients with refractory large B-cell lymphoma. J Adv Pract Oncol. 2019;10(Suppl 3):11-20.
17. Verdun N, Marks P. Secondary cancers after chimeric antigen receptor T-cell therapy. N Engl J Med. 2024;390(7):584-586.
18. FDA. Novel drug approvals for 2023. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2023. Accessed June 30, 2023.
19. van de Donk NWCJ, Zweegman S. T-cell-engaging bispecific antibodies in cancer. Lancet. 2023;402(10396):142-158.
20. Epkinly (epcoritamab-bysp) product information. Plainsboro, NJ: Genmab US, Inc, and North Chicago, IL: AbbVie Inc; May 2023.
21. Columvi (glofitamab-gxbm) product information. South San Francisco, CA: Genentech, Inc; June 2023.
22. ClinicalTrials.gov. Diffuse B-cell lymphoma: search results. https://clinicaltrials.gov/search?cond=Diffuse%20B-Cell%20Lymphoma&aggFilters=status:rec%20act&page=3&limit=100&viewType=Table. Accessed September 30, 2023.
23. PDQ Adult Treatment Editorial Board. Lugano classification for Hodgkin and non-Hodgkin lymphoma [Table 2]. Non-Hodgkin lymphoma treatment (PDQ®): health professional version. In: PDQ Cancer Information Summaries [Internet]. Bethesda, MD: National Cancer Institute; 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





