US Pharm. 2024;49(2):17-26.
ABSTRACT: Cardiovascular disease (CVD) is one of the leading causes of death in the United States. However, medications used to manage CVD are also associated with harm in vulnerable populations. Deprescribing is a systematic process intended to reduce or eliminate medications that are causing more harm than benefit or are unnecessary. Considerations include steps in deprescribing cardiovascular (CV) medications; barriers and facilitators to deprescribing; steps, tools, and strategies to assist with deprescribing of certain classes of CV medications; identifying the impact of deprescribing on mortality, quality of life, adverse drug events, falls, and costs in older adults; and the pharmacist’s role in the deprescribing process.
According to the U.S. Deprescribing Research Network, which is funded through a grant from the National Institute on Aging, deprescribing is “the thoughtful and systematic process of identifying problematic medications and either reducing the dose or stopping these medications in a manner that is safe, effective, and helps people maximize their wellness and goals of care.”1 The term deprescribing was first described in 2003 and was developed to address the impact that polypharmacy had on reducing medication adherence and increasing the risk of adverse drug effects (ADEs) in older adults.2
Cardiovascular Disease Prevalence Among Older Adults
The World Health Organization (WHO) defines cardiovascular disease (CVD) as a group of disorders of the heart and blood vessels that include coronary heart disease, cerebrovascular disease, peripheral arterial disease, rheumatic heart disease, congenital heart disease, deep vein thrombosis, and pulmonary embolism (or thromboembolic disease).3
An interactive summary of data from 2019 to 2021 from the National Health Interview Survey provides insight into the number of older adults with CVD.4 (See TABLE 1.)
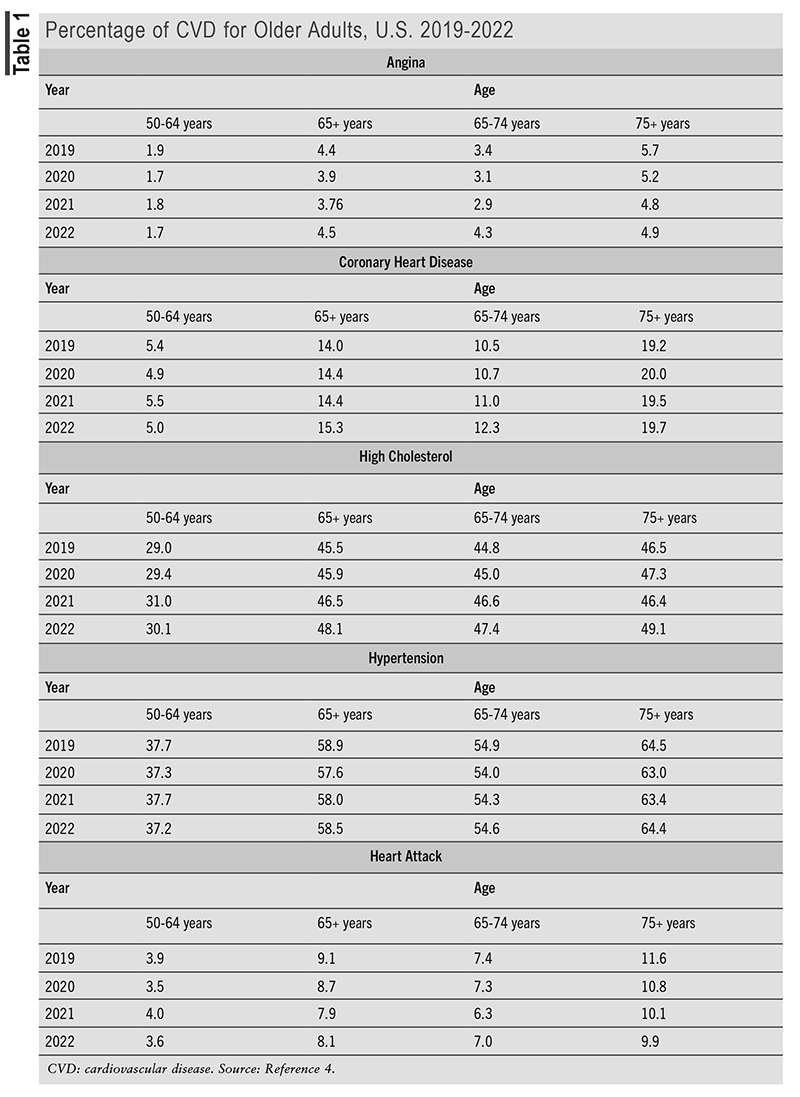
Cardiovascular Prescription Medication Use in Older Adults
Cardiovascular (CV) medications are the most commonly prescribed medication among older adults.5 Data from the National Health and Nutrition Examination Survey, 2015–2016, have shown that among U.S. adults aged 60 to 79 years, three of the five most commonly used prescription medications were CV medications, namely lipid-lowering drugs (45.0%), beta-blockers (22.3%), and angiotensin-converting enzyme inhibitors (ACEIs; 21.3%).6
Harm Associated With CV Agents
A review of 75 studies that investigated the extent of harm caused by CV agents at the time of hospital admission, during hospitalization, upon discharge, and during readmission to the hospital found that CV medications ranked within the top five medications to cause harm, which was often preventable. Antihypertensives and antiarrhythmics were the most common causes of harm. Among the most common ADEs were kidney injury, electrolyte imbalances, and hypotension.7
When to Consider Deprescribing CV Medication
Any discussion regarding deprescribing CV medications, other than in cases of actual medication-related harm, should be conducted using shared decision-making. Factors that need to be considered include patient preferences, life expectancy, clinical status, frailty, comorbidities, drug burden, intent of treatment (primary vs. secondary prophylaxis), availability of deprescribing studies in this population, and patient-centered goals of care. Therapeutic outcomes should focus more on preserving quality of life and less on avoiding long-term complications. This can only be achieved via an individualized approach. Deprescribing should be considered when time-to-benefit exceeds life expectancy, when guideline-associated goals may be burdensome or harmful, in the presence of an ADE, when polypharmacy is present, at the end of life, and in the presence of a prescribing cascade in which another drug is utilized to treat the unrecognized side effects of a previously prescribed medication.8,9
ThinkCascades is a tool for identifying clinically important prescribing cascades that affect older adults. Among the prescribing cascades identified are the use of diuretics to manage calcium channel blocker–induced peripheral edema and the use of urinary antimuscarinic agents to treat diuretic-induced urinary incontinence.10,11
Criteria for Potentially Inappropriate CV Medications in Older Adults
AGS Beers Criteria: The American Geriatrics Society (AGS) Beers Criteria of potentially inappropriate medications (PIMs) in older adults are explicit criteria identifying medications that are best avoided by this group in most circumstances or under specific situations, such as in certain diseases or conditions. The criteria, which were first released in 1991, serve as a tool to deprescribe medications whose risk exceeds their benefits.
The most recent update of the Beers Criteria was released in May 2023 and identified several drugs and drug classes of CV medications that may be potentially inappropriate in an older population.12 (See TABLE 2.)
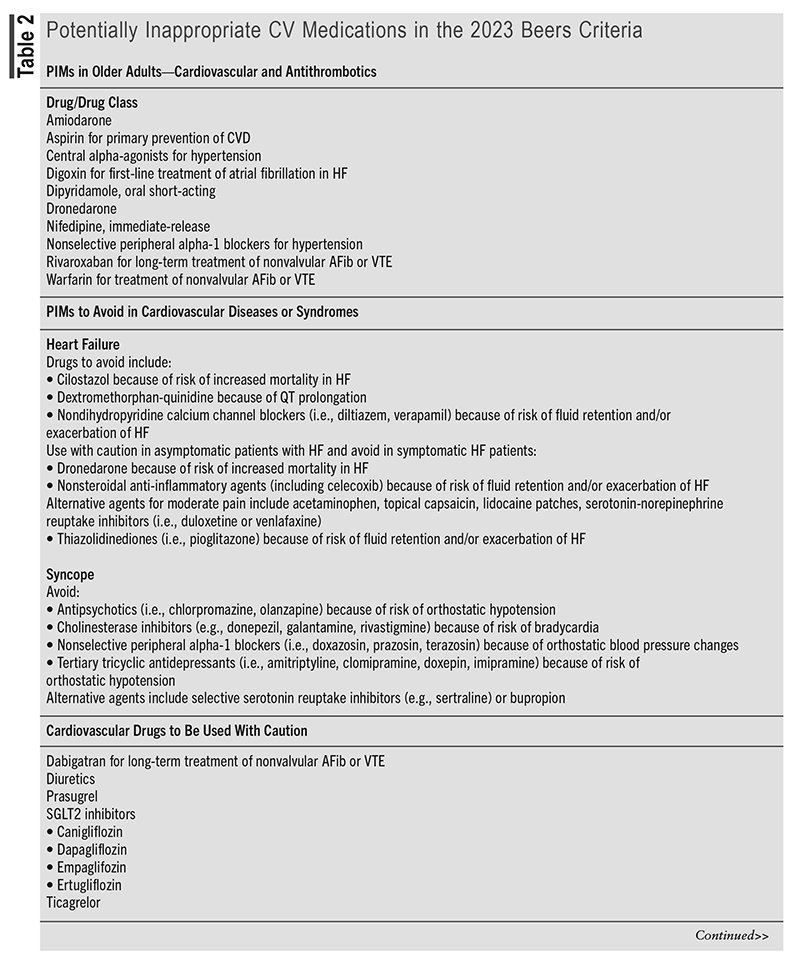
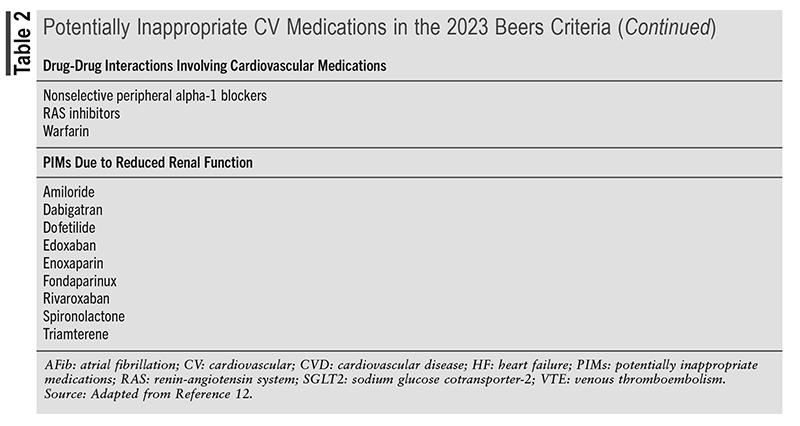
The 2023 AGS Beers Criteria caution that clinicians should consider numerous factors, such as advanced age, cognitive and physical impairment, multicomorbidities, frailty, renal impairment, and polypharmacy, when assessing the risk for harm from PIMs, as individual older adults may have significantly different risks for experiencing a severe medication-related problem. (These recommendations do not apply to patients in palliative or hospice care.) Evaluation of the risk-benefit of PIMs should be in the context of shared decision-making, as it may be reasonable given an older adult’s preferences, values, and treatment goals to initiate or continue a Beers’ list drug.12
STOPP/START Screening Tool: STOPP (Screening Tool of Older Person’s Prescriptions)/START (Screening Tool to Alert to Right Treatment) is a physiological system–based screening tool that uses explicit criteria to indicate which drug or drug classes and under which circumstances a drug or drug class should be stopped or started in an older adult. There are currently three versions of the criteria, with the first published in 2008, the second in 2015, and the most recent in 2023.13-15
The latest version increased STOPP criteria by 61.5% and 23.1% for drugs involving the CV and coagulation systems, respectively. The START criteria increased by 37.5% for drugs involving the CV system, and Version 3 added a new section with two criteria for drugs involving the coagulation system. In Version 3, there are 21 STOPP recommendations for CV agents and 16 STOPP recommendations for coagulation system medications. There are also 11 START recommendations for CV agents and two START recommendations for drugs involved in coagulation. These criteria can be found in the Supplementary Appendix 1 to the STOPP/START Version 3.15,16
STOPPFrail: Modeled after STOPP/START, the STOPPFrail criteria, which are explicit criteria published in 2017, are designed to identify PIM use in frail older adults with limited life expectancy. Of the 27 criteria in the STOPPFrail list, three involve CV and coagulation system medications.17
Version 2 of the STOPPFrail criteria was published in 2020. It includes recommendations on lipid-lowering agents, alpha-blockers, antianginal therapy (e.g., nitrates, ranolazine), and drugs affecting the coagulation system.18
STOPPFall: The STOPPFall (Screening Tool of Older Persons Prescriptions in older adults with high Fall-risk) criteria were developed to identify fall risk–increasing drugs (FRIDs) used in older adults that can be considered for deprescribing. Experts identified 14 medication classes. Among the CV drug classes included were diuretics, alpha-blockers used for either hypertension or prostatic hyperplasia, centrally acting antihypertensives, and vasodilators used in CVD. The criteria provide information on when to consider withdrawal and whether stepwise withdrawal is needed, as well as monitoring parameters.19
IMPACT Tool: IMPACT (Improving Medicines and Polypharmacy Appropriateness Clinical Tool) is a tool developed by the UK National Health System and PrescQIPP, a Community Interest Company, to support the WHO’s campaign of “Medication Without Harm.” The tool helps provide patient-focused care by reviewing the benefits and risk of medications so that clinicians can engage in shared decision-making with their patients. It includes a comprehensive table that identifies drugs and drug classes, considerations to optimize medication use after checking for a valid current indication, and guidance on how to withdraw or taper a medication.
IMPACT provides guidance on deprescribing 14 different drug classes, including medications affecting the CV system. Among the medications in this class are aldosterone antagonists/mineralocorticoid receptor antagonists, antianginals, antiarrhythmics, anticoagulants (oral and injectable), antihypertensives, ACEIs, alpha-1 blockers, central alpha blockers, angiotensin II receptor blockers (ARBs), beta-blockers, calcium channel blockers, diuretics, antiplatelet agents, low-dose aspirin, digoxin, fibrates, nitrates, omega-3 fatty acid supplements, peripheral vasodilators, and statins.20
PRISCUS and PRISCUS 2.0 List: In 2010, a German list of PIMs in older adults called the PRISCUS List was developed. Similar to the Beers Criteria, it used a Delphi consensus method to develop its recommendations. In total, 83 drugs were identified as PIMs. Among them were antiarrhythmics (e.g., flecainide, sotalol, digoxin); platelet aggregation inhibitors/antithrombotics (e.g., ticlopidine, prasugrel); and antihypertensive and CV agents (e.g., clonidine, doxazosin, prazosin, terazosin, methyldopa, non–sustained-release nifedipine). The List also includes possible therapeutic alternatives and precautions to be taken when the drug is used.22
In 2023, PRISCUS 2.0 was published that identified 187 PIMs, the majority (133) of which were not in the original PRISCUS List. Additional CV medications included lidocaine, propafenone as a long-term medication, dronedarone, hydralazine, minoxidil, spironolactone >25 mg/day, pentoxifylline, cilostazol, pindolol, propranolol, and aliskiren.22
Overcoming Barriers to Deprescribing
Identifying medications that are potentially inappropriate in older adults is an essential first step in deprescribing harmful and/or unnecessary drugs. However, there may be barriers to discontinuing these medications. These barriers may be prescriber-focused or patient-focused. It is important to overcome these barriers to achieve successful deprescribing.
Prescriber-Focused Barriers and Facilitators
Prescriber-focused barriers to deprescribing include reluctance to deprescribe CV medications that are not causing ADEs; the presence of a significant past patient history of CV events; patient engagement in an unhealthy lifestyle; evidence of improvement on the CV medication; lack of guideline-directed recommendations on how long therapy should be utilized; when a CV medication was prescribed by a specialist or another prescriber; and when there is a lack of evidence on the safety of withdrawing CV agents. Other prescriber-focused barriers include fear of disease recurrence if a CV medication is deprescribed; when the prescriber perceives that the patient thinks he or she is “giving up”; when patients or their families prefer to stay on a medication; when the prescriber lacks education on deprescribing; when there is lack of financial compensation involved in closely monitoring patients who are being tapered off a medication; when there is poor communication between healthcare professionals regarding medications; and when information is unavailable on how to safely taper or withdraw CV medications.23-26
Prescriber-focused facilitators of deprescribing include when a CV medication is used for primary prevention; when the patient is of advanced age or has a limited life expectancy; if cognitive impairment is present; when persons reside in a long-term care facility or are on palliative/hospice care; and when patients express interest in reducing their medication burden.23-26
Ethical principles of beneficence, nonmaleficence, justice, and autonomy can be used to help guide decisions.8,9
Patient-Focused Barriers and Facilitators
Shared decision-making and communication are essential when deprescribing. The revised Patient Attitudes Toward Deprescribing tool, a 22-question survey, can be used to assess a patient’s attitude toward their medications and their willingness to potentially discontinue or deprescribe a medicine.27
Patient-related barriers to deprescribing CV medications include viewing the medication as a necessity for survival; being frail and viewing the drug as contributing to health; not experiencing an ADE to the medication; fear of experiencing a CV event or of worsening the underlying disease state; prescribers advising against deprescribing; and a lack of understanding about the disease state and the role of medication.23,26,28,29
Patient-related facilitators to deprescribing include having low CV risk or the CVD is under control; discussing potential ADEs with a healthcare professional; being afraid of experiencing an ADE or of becoming dependent on the CV medication; dislike of taking medications; high cost of medications; pride in not having to use medications; viewing medications as poisonous or harmful; having a prescriber who supports deprescribing; having the ability to do a trial off of the medication and adding it back if not feeling well; participating in shared-decision making; and having a complex medication regimen.23,26,28-37
Steps in Deprescribing
There are several tools available that outline the process of deprescribing. Among them are the Five-Step and Seven-Step approaches.9,38,39 The Five-Step method includes the following:
Step 1. Consider all medications currently being taken and the indications for each
Step 2. Evaluate the risk of harm to the patient associated with each medication
Step 3. Assess each medication for the potential to deprescribe it
Step 4. Create a priority list of medications that should be deprescribed before others
Step 5. Implement and monitor the deprescribing regimen.9,38
Another strategy is the Seven-Step medication review method. The elements of this method include the following as they pertain specifically to CV medications39:
Step 1: Determine what matters to the patient
Step 2: Identify essential drug therapy
–Drugs for rate control as well as diuretics and ACEI therapy should be evaluated by an expert before discontinuing when used for left-ventricular systolic dysfunction
–Amiodarone should be discussed with an expert prior to altering the dosage regimen
Step 3: Assess whether the patient is taking unnecessary drug therapy
–Determine if the indication for sodium or potassium supplements is still appropriate
–Determine if there is a valid indication for anticoagulants, anticoagulants + antiplatelet agents, aspirin, dipyridamole, digoxin, peripheral vasodilators, antiarrhythmics, and alpha-blockers
–Assess risk versus benefit for antianginals, antihypertensives, and statins
Step 4: Evaluate the effectiveness of therapy and whether therapeutic objectives are being achieved
–If therapeutic objects are not achieved, consider intensifying antihypertensive therapy for blood pressure control, warfarin therapy if international normalized ratio is subtherapeutic, or rate-limiting drugs if the heart rate is not controlled
–Determine if patients with coronary heart disease might benefit from antithrombotic therapy, statins, ACEI/ARBs, or beta-blockers; if those with previous stroke or transient ischemic attack may benefit from antithrombotics, statins, or ACEI/ARBs; if those with left-ventricular systolic dysfunction might benefit from diuretic therapy, ACEI/ARBs, or beta-blockers; or if those with atrial fibrillation may benefit from antithrombotics or drugs used for rate control
Step 5: Describe whether the patient is at risk of ADEs or is currently experiencing an actual ADE
–Assess safety of drugs that are poorly tolerated in frail adults, which include digoxin at doses >250 mcg
–Assess safety for high-risk medications in a given clinical situation, such as metformin, ACEIs/ARBs, or diuretics in dehydration; concomitant use of a nonsteroidal anti-inflammatory drug (NSAID) and an ACEI/ARB and diuretic or an NSAID and an antithrombotic or the use of NSAIDs, glitazones, or tricyclic antidepressants in patients with congestive heart failure or the use of warfarin along with either a macrolide or quinolone
Step 6: Verify whether the drug therapy is cost-effective
Step 7: Ask if the drug therapy is patient-centered and if the patient is willing and able to take drug therapy as intended
–Assess whether impaired cognition affects self-administration of warfarin or direct-acting oral anticoagulants, as well as the impact of tablet burden.
ARMOR (Assess, Review, Minimize, Optimize, and Reassess) is another tool to assess polypharmacy and to target drugs for potential deprescribing.40
Four steps have been outlined for building deprescribing into the community pharmacy workflow. These steps include capacity building and processes designed to engage patients in deprescribing, which involves educating pharmacists and conducting database reviews to identify potential patients for deprescribing; preliminary interaction to triage patients for possible engagement; detailed interaction with a pharmacist in which a thorough medication history and review are performed and recommendations are made for deprescribing; and follow-up and monitoring.41
Designing a drug regimen around a patient’s pharmacogenomic profile may also be helpful. The Pharmacogenetics Implementation Consortium has disseminated several guidelines that involve CV medications, including clopidogrel (which is metabolized by CYP2C19), warfarin (metabolized by CYP2C9, VKORC1, and CYP4F2), and statins (metabolized by ABCG2 [ATP binding cassette subfamily G member 2], CYP2C9, and SLCO1B1 [solute carrier organic anion transporter family member 1B1]).42-44
Considerations in Deprescribing Specific CV Agents
A systematic review of deprescribing in cardiometabolic conditions in older patients found that the most commonly deprescribed CV medications were statins, antihypertensives, aspirin, digoxin, and diuretics.8
Primary Health Network Tasmania (PHN) is a nongovernment, not-for-profit organization under the Australian government’s Primary Health Networks program that has developed specific deprescribing algorithms for a number of drug classes, including anticoagulants, antihypertensives, antiplatelets, long-acting nitrates, and statins. PHN weighs the risk versus harm of deprescribing, provides a recommended deprescribing strategy, discusses background information on the disease state for which deprescribing is being recommended, reviews the efficacy and adverse effects of medications being considered for deprescribing for a given disease state, discusses factors to consider when deprescribing medications for the focused disease state, and identifies any symptoms that may be expected and are associated with discontinuation of medications for the given disease. Deprescribing is favored when harm exceeds benefit.45 Deprescribing algorithms can be found at www.primaryhealthtas.com.au/resources/deprescribing-resources/.
PHN offers the following algorithms to deprescribe CV agents:
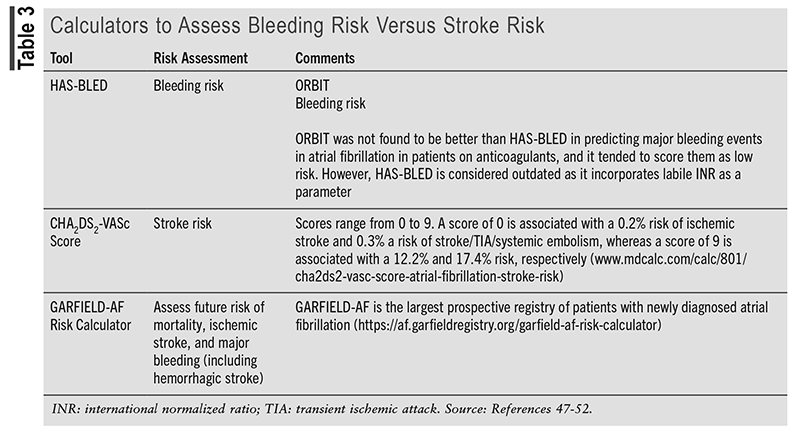
Anticoagulants: See TABLE 3 on Calculators to Assess Bleeding Risk versus Stroke Risk.46-52
Antihypertensives: A Cochrane Database Systematic Review on withdrawal of antihypertensives in older adults found that the evidence was of low or very low certainty to make any conclusions about the effect of deprescribing on all-cause mortality and myocardial infarction.46,53
Antiplatelets: On April 26, 2022, the United States Preventive Services Task Force (USPSTF) strongly recommended against initiating low-dose aspirin for primary prevention of CVD in those aged >60 years. For persons aged 40 to 59 years, the USPSTF advises that the decision to initiate low-dose aspirin for the primary prevention of CVD for those who have a >10% 10-year CVD risk should be individualized. Those who are not at increased risk of bleeding may experience a small net benefit from the use of aspirin as primary prevention of CVD.54,55
The 2023 AGS Beers Criteria also advises against initiating aspirin for primary prevention of CVD due to lack of net benefit and potential for net harm, including risk of major bleeding, and calls on considering deprescribing aspirin in older adults already using aspirin for primary prevention of CVD.12
Long-Acting Nitrates: Deprescribing is recommended in the following groups of patients because of decreased benefit: those with a decrease in myocardial oxygen demand due to a reduction in physical activity; who have undergone revascularization; who are on long-term statin therapy; who have stopped smoking or have made significant lifestyle changes, all of which have improved coronary artery function; and in those whose angina is due to coronary microvascular dysfunction.56
Statins: In August 2022, the USPSTF made the following recommendations about the use of statins in older adults: Clinicians should selectively offer a statin for primary prevention of CVD to adults 40 to 75 years who have >1 CVD risk factor (i.e., dyslipidemia, diabetes, hypertension, smoking) and an estimated 10-year risk of CV event of 7.5% to <10% (although the likelihood of benefit is smaller in this group than in those with a 10-year risk of >10%). For those aged >76 years, the current evidence is insufficient to assess the risk versus benefit in initiating statin therapy for primary prevention of CVD or for mortality.57,58
A critical review on the use of statins in persons aged 70 years and older came out in support of their use after assessing clinical risk, time-to-benefit, and what matters most to the patient.59
A review of guidelines on statins showed that they failed to address the discontinuation of statins in older adults. However, recommendations as to when discontinuation may or may not be appropriate in the presence of intolerance and in light of a patient’s health status (e.g., frailty, limited life expectancy, multimorbidity, functional decline, harm) are applicable to the older adult.60
It is important to keep in mind these algorithms or guidelines on deprescribing should be living documents and should be updated as the science advances and new evidence-based information becomes available.
Deprescribing Impact in Older Adults
In general, the impact of deprescribing medications on mortality, quality of life, adverse events, falls, and/or cost in older adult has been limited and/or mixed. There is much heterogeneity between study populations. Studies are often of low quality; the number and/or types of medications deprescribed differ; frail older adults were often excluded; there is a high risk of bias; and there are methodological issues, such as recruitment difficulties and focusing on discontinuation of medication rather than on clinical or patient-centered outcomes (e.g., drug-related problems, quality of life, mortality, hospital readmissions, falls, or functional status).8,53,61-64
Role of the Pharmacist in Deprescribing
While numerous systematic reviews and/or meta-analyses have explored the role of the pharmacist in deprescribing in various practice settings, few studies have focused on pharmacists’ role in deprescribing CV medications.63,65-74
One study occurred in a hospital setting where pharmacists’ interventions were successful in preventing potential major bleeding from aspirin.73
Another research project occurred in the community pharmacy setting where pharmacists deprescribed cardiometabolic medications. In this study, a pharmacist-led medication review led to 48% of intervention patients having at least one cardiometabolic medication (e.g., insulin, sulfonylurea, diuretic, beta-blocker, statin) either stopped or reduced in dose compared with 31% of control patients (P = .018).74
Despite this encouraging outcome, a systematic review of the role of community pharmacists in deprescribing has yielded mixed results with respect to improvement in quality of life, impact on falls (despite reducing FRIDs), effect on healthcare consumption, and rate of hospitalizations. However, most of the studies included in this review were of short duration (3-12 months). More well-conducted studies are needed.68
Conclusion
Although the science is evolving, when applied to the appropriate population, deprescribing has the potential to reduce harm and cost and improve the quality of life of vulnerable older CV patients. As medication experts, pharmacists can play a major role in identifying patients who are candidates for deprescribing CV agents, assist with the safe dosage reduction or discontinuation of targeted medications, and actively monitor and follow up with these patients to ensure that the benefit of deprescribing outweighs risk.
In memory of Max and Ruby.
REFERENCES
1. US Deprescribing Research Network. https://deprescribingresearch.org/. Accessed December 15, 2023.
2. Woodward MC. Deprescribing: achieving better health outcomes for older people through reducing medications. J Pharm Pract Res. 2003;33:
323-328.
3. World Health Organization. Cardiovascular diseases. www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed December 15, 2023.
4. CDC. Interactive Summary Health Statistics for Adults. wwwn.cdc.gov/NHISDataQueryTool/SHS_adult/index.html. Accessed December 15, 2023.
5. Anfinogenova ND, Trubacheva IA, Popov SV, et al. Trends and concerns of potentially inappropriate medication use in patients with cardiovascular diseases. Expert Opin Drug Saf. 2021;20(10):1191-1206. 6. Hales CM, Servais J, Martin CB, Kohen D. Prescription drug use among adults aged 40–79 in the United States and Canada. NCHS Data Brief, no 347. Hyattsville, MD: National Center for Health Statistics. 2019.
7. Paradissis C, Cottrell N, Coombes I, et al. Patient harm from cardiovascular medications. Ther Adv Drug Saf. 2021;25(12):20420986211027451.
8. Hickman E, Seawoodharry M, Gillies C. Deprescribing in cardiometabolic conditions in older patients: a systematic review. Geroscience. 2023;45(6):3491-3512.
9. Krishnaswami A, Steinman MA, Goyal P, et al. Geriatric Cardiology Section Leadership Council, American College of Cardiology. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol. 2019;73(20):2584-2595.
10. McCarthy LM, Savage R, Dalton K, et al. ThinkCascades: a tool for identifying clinically important prescribing cascades affecting older people. Drugs Aging. 2022;39(10):829-840.
11. Parker R, Henslee A, Cox ZL. A clinical conundrum: the pharmacist's role in evaluating comorbid urinary incontinence and heart failure. Sr Care Pharm. 2021;36(3):142-146.
12. 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081.
13. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83.
14. O'Mahony D, O'Sullivan D, Byrne S. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.
15. O'Mahony D, Cherubini A, Guiteras AR, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023 Aug;14(4):625-632. Erratum in: Eur Geriatr Med. 2023;14(4):633.
16. Appendix 1 in O'Mahony D, Cherubini A, Guiteras AR. STOPP/START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625-632. Erratum in: Eur Geriatr Med. 2023;14(4):633.
17. Lavan AH, Gallagher P, Parsons C, O'Mahony D. STOPPFrail (Screening Tool of Older Persons Prescriptions in Frail adults with limited life expectancy): consensus validation. Age Ageing. 2017;46(4):600-607.
18. Curtin D, Gallagher P, O'Mahony D. Deprescribing in older people approaching end-of-life: development and validation of STOPPFrail version 2. Age Ageing. 2021;50(2):465-471.
19. Seppala LJ, Petrovic M, Ryg J, et al. STOPPFall (Screening Tool of Older Persons Prescriptions in older adults with high fall risk): a Delphi study by the EuGMS Task and Finish Group on Fall-Risk-Increasing Drugs. Age Ageing. 2021;50(4):1189-1199.
20. National Health Service. PrescQIPP Community Interest Company. www.derbyshiremedicinesmanagement.nhs.uk/assets/Clinical_Guidelines/clinical_guidelines_front_page/PrescQipp_IMPACT.pdf. Accessed December 15, 2023.
21. Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int. 201;107(31-32):543-551.
22. Mann NK, Mathes T, Sönnichsen A, et al. Potentially inadequate medications in the Elderly: PRISCUS 2.0. Dtsch Arztebl Int. 2023;120(1-2):3-10.
23. Brunner L, Rodondi N, Aubert CE. Barriers and facilitators to deprescribing of cardiovascular medications: a systematic review. BMJ Open. 2022;12(12):e061686.
24. Abou J, Crutzen S, Tromp V, et al. Barriers and enablers of healthcare providers to deprescribe cardiometabolic medication in older patients: a focus group study. Drugs Aging. 2022;39(3):209-221.
25. Goyal P, Anderson TS, Bernacki GM, et al. Physician perspectives on deprescribing cardiovascular medications for older adults. J Am Geriatr Soc. 2020;68(1):78-86.
26. Luymes CH, van der Kleij RM, Poortvliet RK, et al. Deprescribing potentially inappropriate preventive cardiovascular medication: barriers and enablers for patients and general practitioners. Ann Pharmacother. 2016;50(6):446-454.
27. Reeve E, Low LF, Shakib S, Hilmer SN. Development and validation of the Revised Patients’ Attitudes Towards Deprescribing (rPATD) questionnaire: versions for older adults and caregivers. Drugs Aging. 2016;33(12):913-928.
28. Crutzen S, Baas G, Abou J, et al. Barriers and enablers of older patients to deprescribing of cardiometabolic medication: a focus group study. Front Pharmacol. 2020;11:1268.
29. Goyal P, Requijo T, Siceloff B, et al. Patient-reported barriers and facilitators to deprescribing cardiovascular medications. Drugs Aging. 2020;37(2):125-135.
30. Green AR, Aschmann H, Boyd CM, Schoenborn N. Association between willingness to deprescribe and health outcome priorities among U.S. older adults: results of a national survey. J Am Geriatr Soc. 2022;70(10):2895-2904.
31. George J, Phun YT, Bailey MJ, et al. Development and validation of the medication regimen complexity index. Ann Pharmacother. 2004;38(9):1369-1376.
32. Weir KR, Ailabouni NJ, Schneider CR, et al. Consumer attitudes towards deprescribing: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2022;77(5):1020-1034.
33. Navid P, Nguyen L, Jaber D, et al. Attitudes toward deprescribing among adults with heart failure with preserved ejection fraction. J Am Geriatr Soc. 2021;69(7):1948-1955.
34. Farrell B, Tsang C, Raman-Wilms L, et al. What are priorities for deprescribing for elderly patients? Capturing the voice of practitioners: a modified Delphi process. PLoS One. 2015;10(4):e0122246.
35. Lukacena KM, Keck JW, Freeman PR, et al. Patients’ attitudes toward deprescribing and their experiences communicating with clinicians and pharmacists. Ther Adv Drug Saf. 2022;13:20420986221116465.
36. Green AR, Aschmann H, Boyd CM, Schoenborn N. Assessment of patient-preferred language to achieve goal-aligned deprescribing in older adults. JAMA Netw Open. 2021;4(4):e212633.
37. Crutzen S, Abou J, Smits SE, et al. Older people’s attidudes towards deprescribing cardiometabolic medications. BMC Geriatr. 2021;21(1):366.
38. Takhar S, Nelson N. Deprescribing as a patient safety strategy. Patient Safety Network. Agency for Healthcare Research and Quality. Patient Safety 101. Primer. October 27, 2021. https://psnet.ahrq.gov/primer/deprescribing-patient-safety-strategy#. Accessed December 15, 2023.
39. National Health Service–Scottish Government. 7-steps medication review. https://managemeds.scot.nhs.uk/for-healthcare-professionals/principles/the-7-steps-medication-review. Accessed December 15, 2023.
40. Haque R. ARMOR: a tool to evaluate polypharmacy in elderly persons. Ann Long Term Care. 2009;17(6).
41. Farrell B, Clarkin C, Conklin J, et al. Community pharmacists as catalysts for deprescribing: an exploratory study using quality improvement processes. Can Pharm J (Ott). 2019;153(1):37-45.
42. Clinical Pharmacogenetics Implementation Consortium. CPIC guideline for clopidogrel and CYP2C19. https://cpicpgx.org/guidelines/guideline-for-clopidogrel-and-cyp2c19/. Accessed December 15, 2023.
43. Clinical Pharmacogenetics Implementation Consortium. CPIC guideline for pharmacogenetics-guided warfarin dosing. https://cpicpgx.org/guidelines/guideline-for-warfarin-and-cyp2c9-and-vkorc1/. Accessed December 15, 2023.
44. Clinical Pharmacogenetics Implementation Consortium. CPIC guideline for statins and SLCO1B1, ABCG2, and CYP2C9. https://cpicpgx.org/guidelines/cpic-guideline-for-statins/. Accessed December 15, 2023.
45. Primary Health Tasmania. Medication management–deprescribing. www.primaryhealthtas.com.au/resources/deprescribing-resources/ Accessed December 15, 2023.
46. Primary Health Network Tasmania. A guide to deprescribing anticoagulants. www.primaryhealthtas.com.au/wp-content/uploads/2023/
03/A-guide-to-deprescribing-anticoagulants.pdf. Accessed December 15, 2023.
47. Wang C, Yu Y, Zhu W, et al. Comparing the ORBIT and HAS-BLED bleeding risk scores in anticoagulated atrial fibrillation patients: a systematic review and meta-analysis. Oncotarget. 2017;8(65):109703-109711.
48. Mitchell A, Elmasry Y, van Poelgeest E, Welsh TJ. Anticoagulant use in older persons at risk for falls: therapeutic dilemmas—a clinical review. Eur Geriatr Med. 2023;14(4):683-696.
49. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093-1100.
50. O'Brien EC, Simon DN, Thomas LE, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J. 2015;36(46):3258-3264.
51. Camm AJ, Lip GY, De Caterina R, et al. ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the Management of Atrial Fibrillation: an update of the 2010 ESC Guidelines for the Management of Atrial Fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012 Nov;33(21):2719-2747. Erratum in: Eur Heart J. 2013;34(10):790. Erratum in: Eur Heart J. 2013;34(36):2850-2851.
52. Fox KAA, Lucas JE, Pieper KS, et al. Improved risk stratification of patients with atrial fibrillation: an integrated GARFIELD-AF tool for the prediction of mortality, stroke and bleed in patients with and without anticoagulation. BMJ Open. 2017;7:e017157.
53. Reeve E, Jordan V, Thompson W, et al. Withdrawal of antihypertensive drugs in older people. Cochrane Database Syst Rev. 2020;6(6):CD012572.
54. Primary Health Network [PHN] Tasmania. A guide to deprescribing antiplatelets. www.primaryhealthtas.com.au/wp-content/uploads/2023/03/A-guide-to-deprescribing-antiplatelets.pdf. Accessed December 15, 2023.
55. United States Preventive Services Task Force. Aspirin use to prevent cardiovascular disease: preventive medicine. www.uspreventiveservicestaskforce.org/uspstf/recommendation/aspirin-to-prevent-cardiovascular-disease-preventive-medication. Accessed December 15, 2023.
56. Primary Health Network [PHN] Tasmania. Medication management- deprescribing. Long-acting nitrates. www.primaryhealthtas.com.au/wp-content/uploads/2023/03/A-guide-to-deprescribing-long-acting-nitrates.pdf. Accessed December 15, 2023.
57. Primary Health Network [PHN] Tasmania. Medication management- deprescribing-statins. www.primaryhealthtas.com.au/wp-content/uploads/2023/03/A-guide-to-deprescribing-statins.pdf. Accessed December 15, 2023.
58. United States Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: preventive medicine. Final recommendation statement. August 23, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/statin-use-in-adults-preventive-medication. Accessed December 15, 2023.
59. Hawley CE, Roefaro J, Forman DE, Orkaby AR. Statins for primary prevention in those aged 70 years and older: a critical review of recent cholesterol guidelines. Drugs Aging. 2019;36(8):687-699.
60. van der Ploeg MA, Floriani C, Achterberg WP, et al. Recommendations for (discontinuation of) statin treatment in older adults: review of guidelines. J Am Geriatr Soc. 2020;68(2):417-425.
61. Etherton-Beer C, Page A, Naganathan V, et al. Deprescribing to optimise health outcomes for frail older people: a double-blind placebo-controlled randomised controlled trial-outcomes of the Opti-med study. Age Ageing. 2023;52(5):afad081.
62. Sawan MJ, Moga DC, Ma MJ, et al. The value of deprescribing in older adults with dementia: a narrative review. Expert Rev Clin Pharmacol. 2021;14(11):1367-1382.
63. Thillainadesan J, Gnjidic D, Green S, Hilmer SN. Impact of deprescribing interventions in older hospitalised patients on prescribing and clinical outcomes: a systematic review of randomised trials. Drugs Aging. 2018;35(4):303-319.
64. Anderson LJ, Schnipper JL, Nuckols TK, et al. Members of the PHARM-DC group. A systematic overview of systematic reviews evaluating interventions addressing polypharmacy. Am J Health Syst Pharm. 2019;76(21):1777-1787.
65. Taylor-Rowan M, Alharthi AA, Noel-Storr AH, et al. Anticholinergic deprescribing interventions for reducing risk of cognitive decline or dementia in older adults with and without prior cognitive impairment. Cochrane Database Syst Rev. 2023;12(12):CD015405.
66. Faulkner L, Hughes CM, Barry HE. Interventions to improve medicines optimisation in older people with frailty in primary care: a systematic review. Int J Pharm Pract. 2022;30(4):297-304.
67. Lee JW, Li M, Boyd CM, et al. Preoperative deprescribing for medical optimization of older adults undergoing surgery: a systematic review. J Am Med Dir Assoc. 2022;23(4):528-536.e2.
68. Bužančić I, Kummer I, Držaić M, Ortner Hadžiabdić M. Community-based pharmacists’ role in deprescribing: a systematic review. Br J Clin Pharmacol. 2022;88(2):452-463.
69. Ibrahim K, Cox NJ, Stevenson JM, et al. A systematic review of the evidence for deprescribing interventions among older people living with frailty. BMC Geriatr. 2021;21(1):258.
70. Dills H, Shah K, Messinger-Rapport B, et al. Deprescribing medications for chronic diseases management in primary care settings: a systematic review of randomized controlled trials. J Am Med Dir Assoc. 2018;19(11):923-935.e2.
71. Chan M, Plakogiannis R, Stefanidis A, et al. Pharmacist-led deprescribing for patients with polypharmacy and chronic disease states: a retrospective cohort study. J Pharm Pract. 2023;36(5):1192-1200.
72. Le V, Patel N, Nguyen Q, et al. Retrospective analysis of a pilot pharmacist-led hospice deprescribing program initiative. J Am Geriatr Soc. 2021;69(5):1370-1376.
73. Scarfo NL, Thomas V, Mayboroda MS, Hewage U. Aspirin deprescribing in primary prevention of cardiovascular disease: a prospective risk-benefit approach. Intern Med J. 2023;53(11):2007-2015.
74. Crutzen S, Baas G, Denig P, et al. Pharmacist-led intervention aimed at deprescribing and appropriate use of cardiometabolic medication among people with type 2 diabetes. Res Social Adm Pharm. 2023;19(5):783-792.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






