ABSTRACT: Post-transplant diabetes mellitus (PTDM) is a possible consequence following solid-organ transplantation. Patients with PTDM are at risk for complications associated with traditional diabetes mellitus (DM), including infection, cardiovascular events, and graft failure. Risk factors include both transplant-related factors and those for type 2 DM. The immunosuppressive medications used after transplantation are a common risk factor for PTDM. The immunosuppressive agent belatacept is a potential alternative for reducing the risk of PTDM. Patients who undergo a kidney transplant may face limitations in treatment options for DM or PTDM because of the uncertainty of their renal function post transplantation. Pharmacists can play a role in PTDM awareness and can recommend appropriate therapies for these patients.
Post-transplant diabetes mellitus (PTDM) is a possible consequence following solid-organ transplantation. Patients should be aware of the risk of PTDM, as it increases their risk of graft failure.1 Although kidney transplants improve patient survival compared with long-term dialysis, complications following transplants remain a concern. PTDM is a significant risk factor for cardiovascular (CV) disease and chronic kidney disease (CKD) following transplant.2 Patients may develop new-onset diabetes mellitus (DM) post transplantation or have undiagnosed DM prior to transplantation. The term new-onset diabetes post-transplantation (NODAT) is therefore thought to be misleading, and this article will refer to PTDM.3 Common immunosuppressive regimens following transplant can increase a patient’s risk of developing PTDM and may lead to barriers in management. Immunosuppressive therapies that increase the risk of PTDM include calcineurin inhibitors (CNIs), corticosteroids, and mammalian target of rapamycin (mTOR) inhibitors. Belatacept is a potential option for decreasing the risk of hyperglycemia in this patient population. This article aims to provide an overview on the diagnosis, complications, and treatment of PTDM.
Risk Factors
Risk factors for PTDM include transplant-related as well as traditional risk factors for type 2 DM (T2DM). Common traditional pretransplant risk factors include age (>40 years), BMI (>25 kg/m2), male sex, and family history. Patients undergoing kidney transplantation have additional transplant-related risk factors for developing DM.4 Transplant-related risk factors include the use of various immunosuppressive agents employed post transplant. The immunosuppressive therapies include corticosteroids, CNIs, and mTOR inhibitors. Corticosteroids have a dose-dependent effect in causing hyperglycemia by inducing insulin resistance and increasing hepatic gluconeogenesis.5 CNIs such as tacrolimus and cyclosporine also increase the risk of PTDM. Calcineurin is an important factor in beta-cell function, and when calcineurin is inhibited, secretion of insulin from beta cells is decreased. Tacrolimus is known to have an increased risk over cyclosporine.4 mTOR inhibitors such as sirolimus are also thought to contribute to PTDM by blocking beta-cell proliferation as well as insulin production and secretion.6 Mycophenolate mofetil and azathioprine are not associated with PTDM. Additional risk factors post transplant include immunosuppression and infection, specifically hepatitis C virus or cytomegalovirus (CMV). Weight gain is also common and increases the risk of developing PTDM.4
Epidemiology
The incidence of PTDM has been reported in 9.1% of patients at 3 months, 16% at 12 months, and 24% at 36 months post transplant. Most organ recipients (76.5%) develop PTDM in the first 3 months following solid-organ transplant.7 The incidence of PTDM has been estimated to occur in 4% to 25% of kidney-transplant, 2.5% to 25% of liver-transplant, 4% to 40% of heart-transplant, and 30% to 35% of lung-transplant recipients.3 Patients who are considered high-risk recipients are at even greater risk for developing PTDM. It is estimated that about 50% of high-risk kidney-transplant recipients will develop PTDM.2
Diagnosis
Diagnosis of PTDM is described in the 2014 International Consensus Guidelines on Screening, Diagnosis, and Management of PTDM.8 The guidelines suggest that patients with hyperglycemia early post transplantation should not be diagnosed with PTDM. PTDM diagnosis is made when a patient is stable on maintenance immunosuppressive therapy 3 months following transplant. However, glycosylated hemoglobin (HbA1c) testing alone <365 days following transplantation will often underestimate PTDM, and other tests should also be performed (oral glucose tolerance test [OGTT] is preferred). The timeline for PTDM diagnosis is outlined in TABLE 1.
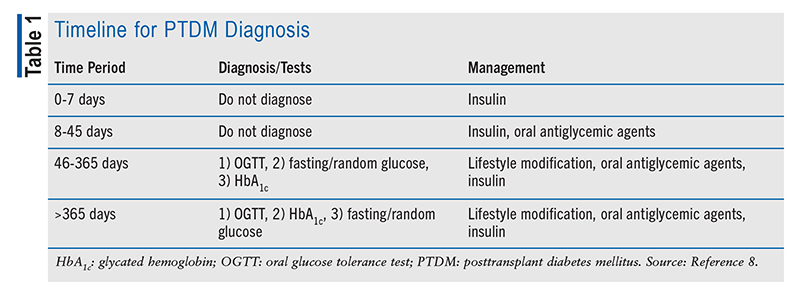
To diagnose PTDM, a patient must have one of the following: a random plasma glucose >200 mg/dL (11.1 mmol/L) plus symptoms of DM (polyuria, polydipsia, weight loss, tiredness), fasting plasma glucose >126 mg/dL (7.0 mmol/L), 2-hour plasma glucose following 75 g oral glucose ≥200 mg/dL during an OGTT, or HbA1c >6.5%. Prediabetes is defined as either a fasting blood glucose of 100 to 126 mg/dL (5.6-6.9 mmol/L) or fasting plasma glucose <7.0 mmol/L plus 2-hour plasma glucose following 75 g oral glucose of 7.8 to 11.00 mmol/L. Patients with HbA1c 5.7% to 6.4% are considered to have an increased risk of PTDM.8
Complications
Patients with PTDM are at risk for complications that are associated with traditional DM, but at an accelerated pace. Additionally, these patients have a higher risk of infections as well as CV events. Patients who are prescribed immunosuppressive therapies are predisposed to many types of infections and have a greater risk of sepsis and sepsis-related mortality.7 Increased fasting glucose levels at 1, 4, and/or 12 months post transplant are significantly related to CV events, independent of other CV risk factors.9 Specifically, fasting glucose levels >100 mg/dL are associated with higher incidences of post-transplant cardiac and peripheral vascular disease events.9 PTDM is also associated with reduced graft survival, which can lead to an increased risk of infection and an increased incidence of mortality.
Treatment Overview
Medication Management: According to the 2014 International Consensus Guidelines on Screening, Diagnosis, and Management of PTDM, patients with early post-transplant hyperglycemia (defined as hyperglycemia in days 0-46 post transplant) should be monitored and not diagnosed with PTDM.8 As the doses of immunosuppressive medications are gradually reduced, hyperglycemia may improve or resolve.1 The higher doses of immunosuppressive medications used immediately after transplant may correlate to a higher incidence of hyperglycemia.7 If persistent hyperglycemia occurs in days 0 through 7 post transplant, insulin may be used. Insulin or oral antiglycemic agents are recommended in days 8 through 45 post transplant. Once patients are 46 days post transplant, and once patients are on the likely maintenance immunosuppressive regimen, PTDM may be managed through lifestyle modification, oral antiglycemic agents, and insulin. The management and treatment goals for PTDM are determined according to the most recent American Diabetes Association (ADA) Standards of Care in Diabetes. Patients should target an HbA1c of 6.5% to 7%; however, this goal may be individualized according to age, comorbidities, ability to self-manage, and patient preference.
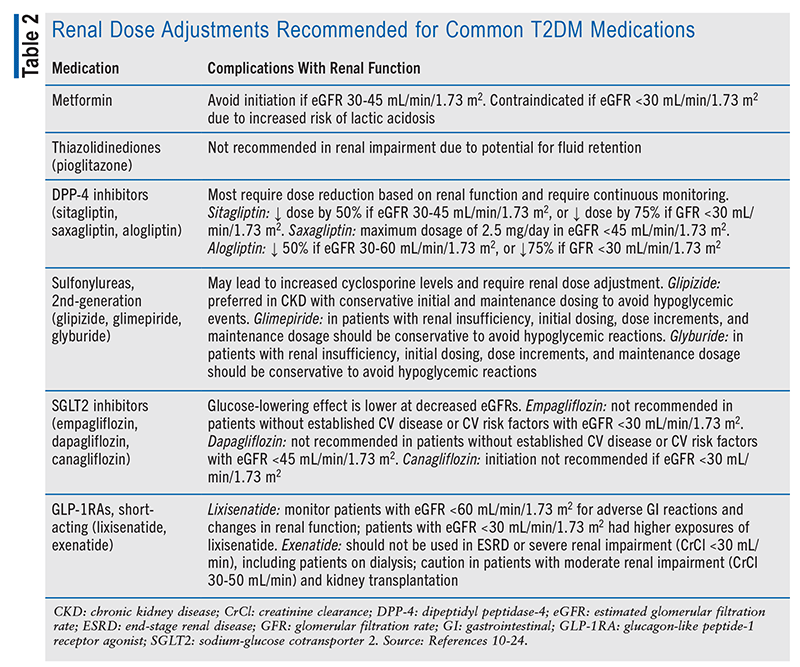
Patients who undergo kidney transplantation may have potential barriers to medications based on their renal function post transplant. TABLE 2 outlines the renal dose adjustments recommended for common medications used for T2DM.10-24 Owing to the uncertainty of a patient’s response to the transplant, agents that do not require renal adjustment may be preferred over other agents. This includes glucagon-like peptide-1 receptor agonists (GLP-1RAs), including dulaglutide, liraglutide, and semaglutide, and the dipeptidyl peptidase-4 inhibitor linagliptin. Agents that have a CV benefit should also be considered because of the increased risk for CV events in these patients. Per the 2023 ADA Standards of Care in Diabetes guidelines, preferred agents to reduce CV risk in patients with CKD include a sodium-glucose cotransporter 2 (SLGT2) inhibitor or a GLP-1RA with proven CV disease benefit if an SGLT2 cannot be tolerated or is contraindicated.11 Both of these agents are renally protective and help maintain renal function, making them beneficial for patients who have received a kidney transplant. As mentioned above, insulin is also an option in these patients, despite the increased risk of hypoglycemia. The kidneys clear 30% to 80% of insulin; therefore, with reduced renal function, insulin will have a prolonged half-life.25 Renal function must be monitored to determine the proper insulin requirements to prevent hypoglycemic events.
Lifestyle: Lifestyle recommendations for PTDM are based on the 2023 ADA Standards of Care in Diabetes guidelines for patients with T2DM. Recommendations include incorporating nutritional changes, increasing physical activity, and using behavioral approaches to assist with blood glucose management.26 For nutritional changes, patients are encouraged to target an energy deficit of 500 kcal/day to 750 kcal/day to assist with weight loss. Some recommended diets include the Dietary Approaches to Stop Hypertension eating pattern, which includes reducing sodium consumption to <2 g/day and increasing potassium intake, and the Mediterranean diet, which includes lowering saturated and trans fat intake while increasing plant stanols/sterols, n-3 fatty acids, and viscous fiber.26,27 With regard to exercise, all patients should perform >150 minutes of moderate-intensity aerobics per week.26 Patients who cannot achieve this level of exercise initially should start by exercising for a shorter time and/or for fewer days and then work up to this goal. Behavioral interventions, which are used to encourage nutritional and exercise changes, may include individual or group counseling sessions. During these sessions, the patient’s motivation level, life circumstances, and willingness to implement behavioral changes should be assessed to determine the likelihood of disease-state improvement.26
Belatacept
Belatacept is a fusion protein composed of the human immunoglobulin G1 Fc fragment linked to the modified extracellular domain of cytotoxic T lymphocyte–associated antigen. Belatacept selectively inhibits T-cell activation by blocking the CD28-CD80/86 costimulatory pathway and is indicated post kidney transplant for prophylaxis of organ rejection as an alternative to CNIs.28
A phase IIIb randomized clinical trial demonstrated a favorable benefit-risk ratio for conversion to belatacept 6 to 60 months post transplantation compared with continuation of CNI therapy in stable kidney-transplant recipients.28 CNIs, the current standard of care after kidney transplantation, are potentially nephrotoxic, and long-term use may lead to increased rates of late graft rejection, CV events, and NODAT compared with other treatments.29
In the phase IIIb trial, patients either continued CNI therapy post kidney transplant or converted to belatacept. All patients continued to receive mycophenolate (mofetil or sodium salt) and daily corticosteroids and were followed for 24 months. The primary outcome, the percentage of patients surviving with a functioning graft at 24 months, was similar between the groups. However, the secondary outcome, patients with cellular (Banff >1A) or antibody-mediated biopsy-proven acute rejection (BPAR), as well as mean adjusted change from baseline at month 24 in estimated glomerular filtration rate in mL/min/1.73 m2, was higher in the belatacept group. Although the belatacept group had a higher BPAR rate, all rejection events occurred in the first 6 to 7 months and responded to treatment, whereas in the CNI group, rejection events occurred over the course of 2 years.28
Safety monitoring of patients for serious viral infections, including infection with CMV or human polyomavirus 1 (BK virus), was performed throughout the phase IIIb trial and at follow-up, 8 weeks after the last dose.28 Safety monitoring was not specified in the protocol and was performed at the discretion of individual investigators. CMV occurred in two patients in the belatacept group and in zero patients in the CNI group. Conversion from CNI-based therapy to belatacept is associated with an increased risk of CMV, which is also a listed risk factor for PTDM.30
No current studies have directly compared the reduction in risk of PTDM with CNIs versus belatacept; however, belatacept does lessen the risk of nephrotoxicity and therefore reduces limitations to the common medications used to treat DM. In addition, according to the FDA package inserts, CNIs carry an adverse effect of hyperglycemia, whereas belatacept does not. Tacrolimus has a risk of >40%, and cyclosporine has a risk of <2%.31,32 Belatacept does not list hyperglycemia as an adverse effect.33
A meta-analysis that evaluated belatacept in kidney-transplant recipients concluded that belatacept was associated with a 39% lower incidence of diabetes versus treatment with a CNI (four studies, 1,049 recipients; risk ratio 0.61, 95% CI, 0.40-0.93). The meta-analysis additionally concluded that patients treated with belatacept post kidney transplant are less likely to have renal scarring and more likely to have an increased glomerular filtration rate, lower blood pressure (systolic and diastolic), and a better lipid profile.34
Overall, belatacept is an alternative option for patients with DM after kidney transplantation. Based on its decreased risk of nephrotoxicity, as well as its adverse-effect profile and tolerability, clinicians may consider belatacept in order to improve patient outcomes in PTDM.
The Pharmacist’s Role
Pharmacists play an important role in the prevention and treatment of PTDM. Patients receiving a solid-organ transplant should be screened for the potential development of PTDM. Pharmacists have the ability to assess patients’ risk factors and identify those who should be monitored and placed on therapy for PTDM. For patients who are on immunosuppressive or steroid regimens post transplantation, blood glucose levels should be checked regularly. Pharmacists as well as other healthcare providers can recommend, monitor, and adjust therapies to achieve the best possible outcome for patients. Furthermore, pharmacists can recommend appropriate therapies to address patients’ additional comorbidities that may contribute to increased risks of morbidity and mortality post transplantation.
Conclusion
PTDM is possible following kidney transplantation, and all recipients should be aware of this complication to reduce their risk of graft failure. Transplant-specific factors, especially immunosuppressive medications, are leading factors in the development of PTDM. Pharmacists can play a key role in promoting awareness of PTDM, and they can recommend screening options for patients who may be at increased risk and recommend appropriate therapies. The common medications used for PTDM may pose limitations in this patient population with regard to renal function. Belatacept is a potential alternative for reducing the risk of PTDM that pharmacists can recommend based on the patient’s specific risk factors.
REFERENCES
1. Kuo HT, Sampaio MS, Vincenti F, Bunnapradist S. Associations of pretransplant diabetes mellitus, new-onset diabetes after transplant, and acute rejection with transplant outcomes: an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) database. Am J Kidney Dis. 2010;56(6):1127-1139.
2. Montero N, Oliveras L, Soler MJ, Cruzado JM. Management of post-transplant diabetes mellitus: an opportunity for novel therapeutics. Clin Kidney J. 2021;15(1):5-13.
3. Pham PT, Sarkar M, Pham PM, Pham PC. Diabetes mellitus after solid organ transplantation. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc; 2000-.
4. Chowdhury TA. Post-transplant diabetes mellitus. Clin Med (Lond). 2019;19(5):392-395.
5. Shivaswamy V, Boerner B, Larsen J. Post-transplant diabetes mellitus: causes, treatment, and impact on outcomes. Endocr Rev. 2016;37(1):37-61.
6. Rovira J, Ramírez-Bajo MJ, Banon-Maneus E, et al. mTOR inhibition: reduced insulin secretion and sensitivity in a rat model of metabolic syndrome. Transplant Direct. 2016;2(2):e65.
7. Solhjoo M, Kumar SC. New onset diabetes after transplant. Treasure Island, FL: StatPearls Publishing; 2023 Jan-.
8. Sharif A, Hecking M, de Vries AP, et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14(9):1992-2000.
9. Cosio FG, Kudva Y, van der Velde M, et al. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005;67(6):2415-2421.
10. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):s1-s155.
11. ElSayed NA, Aleppo G, Aroda VR, et al; American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Care in Diabetes—2023. Diabetes Care. 2023;46(Suppl 1):s140-s157.
12. Glucophage (metformin hydrochloride) product information. Princeton, NJ: Bristol-Myers Squibb Co; April 2017.
13. Actos (pioglitazone hydrochloride) product information. Deerfield, IL: Takeda Pharmaceuticals America, Inc; July 2011.
14. Januvia (sitagliptin) product information. Rahway, NJ: Merck Sharp & Dohme LLC; July 2023.
15. Onglyza (saxagliptin) product information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; July 2009.
16. Nesina (alogliptin) product information. Deerfield, IL: Takeda Pharmaceuticals America, Inc; January 2013.
17. Glucotrol (glipizide) product information. New York, NY: Pfizer Inc; August 2023.
18. Amaryl (glimepiride) product information. Bridgewater, NJ: sanofi-aventis U.S. LLC; October 2013.
19. DiaBeta (glyburide) product information. Bridgewater, NJ: sanofi-aventis U.S. LLC; July 2009.
20. Jardiance (empagliflozin) product information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; September 2023.
21. Farxiga (dapagliflozin) product information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; April 2021.
22. Invokana (canagliflozin) product information. Titusville, NJ: Janssen Pharmaceuticals, Inc; July 2023.
23. Adlyxin (lixisenatide) product information. Bridgewater, NJ: sanofi-aventis U.S. LLC; July 2016.
24. Byetta (exenatide) product information. Princeton, NJ: Bristol-Myers Squibb Co; November 2014.
25. Hahr AJ, Molitch ME. Management of diabetes mellitus in patients with chronic kidney disease. Clin Diabetes Endocrinol. 2015;1:2.
26. ElSayed NA, Aleppo G, Aroda VR, et al; American Diabetes Association. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: Standards of Care in Diabetes—2023. Diabetes Care. 2023;46(Suppl 1):s128-s139.
27. ElSayed NA, Aleppo G, Aroda VR, et al; American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Care in Diabetes—2023. Diabetes Care. 2023;46(Suppl 1):s158-s190.
28. Budde K, Prashar R, Haller H, et al. Conversion from calcineurin inhibitor- to belatacept-based maintenance immunosuppression in renal transplant recipients: a randomized phase 3b trial. J Am Soc Nephrol. 2021;32(12):3252-3264.
29. Farouk SS, Rein JL. The many faces of calcineurin inhibitor toxicity—what the FK? Adv Chronic Kidney Dis. 2020;27(1):56-66.
30. Karadkhele G, Hogan J, Magua W, et al. CMV high-risk status and posttransplant outcomes in kidney transplant recipients treated with belatacept. Am J Transplant. 2021;21(1):208-221.
31. Prograf (tacrolimus) product information. Deerfield, IL: Astellas Pharma US, Inc; February 2012.
32. Gengraf (cyclosporine) product information. North Chicago, IL: AbbVie Inc; February 2021.
33. Nulojix (belatacept) product information. Princeton, NJ: Bristol-Myers Squibb Co; April 2018.
34. Masson P, Henderson L, Chapman JR, et al. Belatacept for kidney transplant recipients. Cochrane Database Syst Rev. 2014;(11):CD010699.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





