US Pharm. 2024;49(3):59-60.
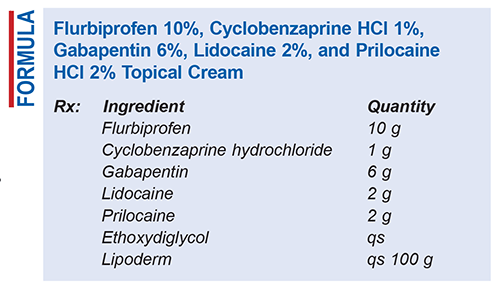
Method of Preparation: Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh/measure each ingredient. In a mortar, triturate all of the powders to a fine, homogenous mixture. Incorporate a few drops of ethoxydiglycol and mix, ensuring even dispersion, until a smooth paste forms. Geometrically, incorporate the Lipoderm to final weight and mix to a homogenous cream. Package and label.
Use: This topical preparation may be used to relieve pain from conditions such as neuropathy, fibromyalgia, muscle spasms, arthritis, and tendonitis.
Packaging: Package in a tight, light-resistant container at controlled room temperature.
Labeling: Keep out of reach of children. Use only as directed. Store in a cool, dry place. Avoid direct sunlight. Discard after [time period].1
Stability: The USP default beyond-use date for this preparation is up to 180 days when stored at room temperature.2 (Note: Stability studies might be conducted that will explore extension of the beyond-use date.)
Quality Control: Weight/volume, pH, specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, and physical stability (discoloration, foreign materials, gas formation, mold growth) are examples of quality-control assessments.2-4
Discussion: Flurbiprofen (Ansaid, C15H13FO2, MW 244.26) is a white or slightly yellow, crystalline powder belonging to the phenylalkanoic acid derivative group of nonsteroidal anti-inflammatory drugs. Comprising a racemic mixture of (+)S- and (–)R-enantiomers, it is slightly soluble in water at a pH of 7.0, and it readily dissolves in most polar solvents. Flurbiprofen possesses analgesic, anti-inflammatory, and antipyretic properties. It inhibits cyclooxygenase-1 and -2 and decreases prostaglandin synthesis in peripheral tissues.5
Cyclobenzaprine hydrochloride (C20H21N • HCl, MW 311.9) is a white, crystalline tricyclic amine salt. It is highly soluble in water and alcohol, sparingly soluble in isopropanol, and insoluble in hydrocarbon solvents. If aqueous solutions are made alkaline, the free base will separate. Cyclobenzaprine HCl alleviates skeletal muscle spasm without interfering with muscle function.6
Gabapentin (Neurontin, cyclohexaneacetic acid, C9H17NO2, MW 171.24) is a white to off-white, crystalline solid that is freely soluble in water and in alkaline and acidic solutions. Gabapentin is an anticonvulsant that is structurally related to gamma-aminobutyric acid. Gabapentin is used in combination with other anticonvulsants to manage seizure disorders, neuropathic pain, vasomotor symptoms, and various other disorders.7
Lidocaine (C14H22N2O • HCl, MW 234.3) is a white, crystalline, odorless powder with a slightly bitter taste. Lidocaine is in the amide-type class of local anesthetics. It is highly soluble in water (1:0.7) and alcohol (1:1.5), and its pKa is 7.86.8
Prilocaine (C13H20N2O, MW 220.3) is a white, crystalline, odorless powder with a sour, bitter taste that is soluble in water and ethanol and slightly soluble in chloroform. An amide-type local anesthetic and anticonvulsant, prilocaine is used for infiltration anesthesia, peripheral nerve block, and spinal and epidural anesthesia. It has a rapid onset and an intermediate duration of action. Prilocaine is commonly combined with lidocaine in topical products for dermal anesthesia.8,9
Ethoxydiglycol (CH2OHCH2OCH2CH2OC2H5, C6H14O3, MW 134.20) is also known as diethylene glycol monoethyl ether, diethylene glycol ethyl ether, Transcutol, and Carbitol. It occurs as a colorless liquid with a mild odor and has a density of 1.0272. Ethoxydiglycol, a hygroscopic organic solvent, can be mixed with water as well as with other organic solvents. Ethoxydiglycol improves the solubility and permeability of active ingredients and functions as a solubilizer and cosurfactant. Ethoxydiglycol is nonpenetrating and nonirritating.10
Lipoderm is a transdermal base with a smooth, creamy texture lacking the tackiness of Pluronic Lecithin Organogel, and its consistency is less greasy. Lipoderm contains a proprietary liposomal component that enhances the permeation of various active substances. Lipoderm’s formulation provides a stable transdermal delivery system that can be used with a variety of therapeutic agents.11
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; September 2022.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of ointments/creams/gels. IJPC. 1998;2:308-309.
3. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 1: physical and chemical testing. IJPC. 2019;23:211-216.
4. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 2: microbiological testing. IJPC. 2019;23:299-303.
5. Ansaid (flurbiprofen) product information. New York, NY: Pfizer Inc; May 2016.
6. Cyclobenzaprine hydrochloride product information. Miami, FL: Cipla USA Inc; July 2016.
7. Sweetman SC, ed. Martindale: The Complete Drug Reference. 36th ed. London, England: Pharmaceutical Press; 2009:482-484.
8. White HS. Local anesthetics. In: Gennaro AR, ed. Remington: The Science and Practice of Pharmacy. 20th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2000:1402-1403.
9. DrugBank Online. Prilocaine. https://go.drugbank.com/drugs/DB00750. Accessed February 2, 2024.
10. Ash M, Ash I. Handbook of Pharmaceutical Additives. Brookfield, VT: Gower Publishing Ltd; 1995:484.
11. PCCA. Lipoderm. www.pccarx.com/products/PCCALIPODERM%C2%AE/30-3338/PROPRIETARYBASES. Accessed February 2, 2024.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





