US Pharm. 2024;49(1):37-41.
ABSTRACT: Specialty medications are used to treat complex disease states and may require additional expertise in handling and administration. As more patients require access to these high-cost medications, pharmacists with specialty pharmacy expertise positively impact this experience for both patients and prescribers. Such pharmacy teams are able to circumvent insurance barriers, such as prior authorizations and network restrictions, improve adherence rates, and contribute to healthcare cost savings.
Specialty pharmacy has garnered a vital role in healthcare because several factors have altered the healthcare landscape. In recent years, there has been a rapid increase in the development and availability of specialty medications, which are often high-cost therapies designed to treat complex conditions. Specialty pharmacies provide personalized care to ensure that patients with rare, complex medical conditions and/or those receiving high-cost therapies receive effective and tailored treatment.1,2
The National Association of Specialty Pharmacy defines specialty pharmacy as a state-licensed pharmacy that solely or largely provides medications for people with serious health conditions requiring complex therapies.1 In-depth monitoring of these therapies for safety and efficacy is key to patient-centered care. These intricacies require expert oversight to ensure that patients can afford, obtain, and initiate therapy in a timely manner, be closely monitored for any adverse events or changes in health that require prompt intervention, and receive appropriate therapy. Specialty medications account for 55% of U.S. medication spending, and specialty patients may incur more total healthcare costs than nonspecialty patients due to disease relapse, flare-ups, or disease progression.3,4
What Are Specialty Medications?
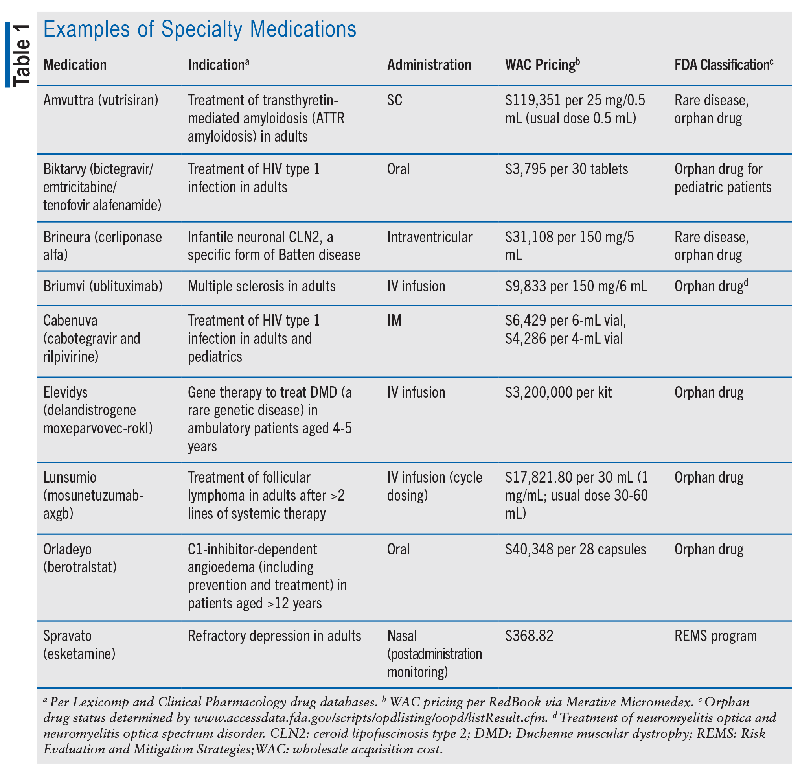
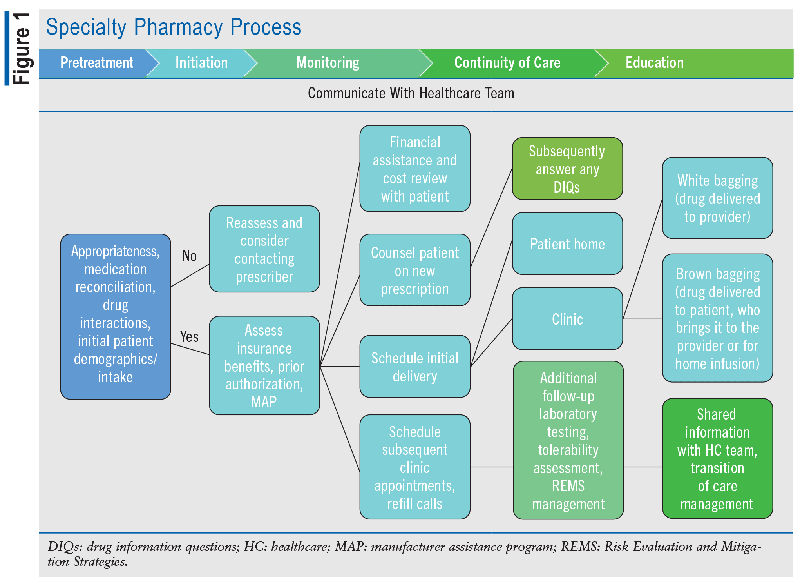
Specialty medications represent a distinct category of therapies that are significantly more complex than most prescription medications, particularly in their application and administration.1 These specialized therapies treat severe and often life-threatening conditions, such as cancer, autoimmune disorders, or rare genetic diseases. In comparison with most prescription medications, specialty therapies entail a multitude of unique challenges. They often demand specialized distribution and delivery procedures due to their stringent storage requirements or necessity for comprehensive patient education and monitoring.3 Additionally, the cost of these medications can be notably high, owing to their research and development expenses and limited treatment populations. Some specialty medications serve as vital therapies for rare diseases that affect a small number of individuals. Such medications may also qualify as orphan medications, which provide regulatory benefits and incentives to promote their development and accessibility for patients facing these less common, yet equally critical, medical challenges. Overall, specialty medications constitute a crucial but intricate component of the pharmaceutical landscape, requiring specialized attention and resources to ensure their effective utilization in improving patients’ lives. TABLE 1 lists some examples of specialty medications, and FIGURE 1 describes the specialty pharmacy process.
The Role of Specialty Pharmacies
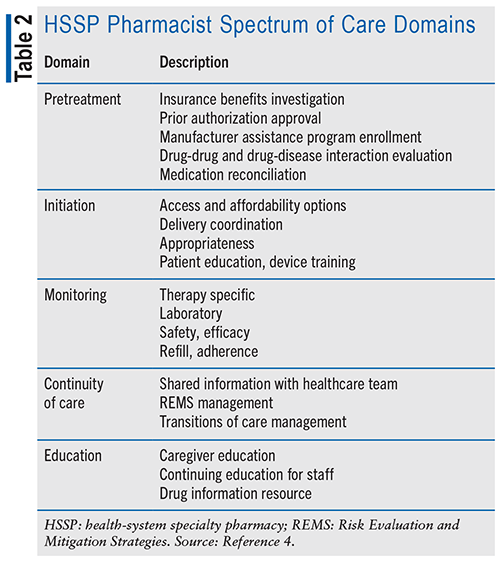
There are many different types of specialty pharmacies, including centralized mail-in, independently owned or chain pharmacies, and health-system specialty pharmacies (HSSPs). Payer-owned specialty pharmacies are facilities or organizations that are operated and owned by pharmacy benefit managers. These pharmacies are typically established to enhance the management and cost control of specialty medications, elevate patient outcomes, and optimize the overall healthcare process. Some examples of payer-owned specialty pharmacies include OptumRx (subsidiary of UnitedHealth Group), Cigna Specialty Pharmacy, Express Scripts Specialty Pharmacy (part of Cigna), CVS Health Specialty Pharmacy, and Humana Specialty Pharmacy.5 These payer-owned specialty pharmacies aim to integrate prescription drug management and patient care more closely, with the goal of improving outcomes, reducing costs, and ensuring that patients receive necessary support for their complex medical needs. Specialty pharmacies owned by hospitals, health systems, and physician practices have steadily increased in number. Their market presence expanded from 11% in 2015 to 39% in 2020.6 HSSPs provide specialized pharmaceutical services to patients within a health system.4 Pharmacists integrated into specialty clinics have direct access to providers and electronic health records. For patients, this collaborative practice model allows for uninterrupted pharmaceutical care and close monitoring for safety and efficacy of these high-cost therapies. The spectrum of care from an HSSP pharmacist has been broken down into five proposed domains.4 TABLE 2 describes these domains.
Patient-Centered Care
Studies demonstrated positive impacts of specialty pharmacists on patient care for both clinical and nonclinical outcomes, such as cost.4,7-11 Patients with HIV who used an HSSP (n = 46) showed increased antiretroviral adherence at 6 months compared with patients who did not use an HSSP (n = 50), although viral suppression and CD4 counts did not differ significantly between groups.7 Patients using a HSSP were more likely to have adherence rates >90% (89.1% vs. 61%, P <.01). Although more patients achieved viral load <20 copies/mL and CD4 counts >200 cells/μL with an HSSP compared with without (93.5% vs. 82%, P = .12, and 97.8% vs. 92%, P = .36, respectively), these differences were not statistically significant between groups. A review of 20 studies of various medication classes found that when an HSSP was used, the proportion of days covered and medication possession ratio ranged from 89% to 100%, which is higher than the general target of >80% for most disease states set by Pharmacy Quality Alliance (a nonprofit organization that develops and implements medication quality measures to assess and quantify the quality of medication use, aiming to optimize patient outcomes).4,12 A virtual pharmacy consult service for patients (n = 157) requiring biological therapy in a rheumatology clinic demonstrated that 41% required an intervention to complete safety monitoring.8 Additionally, the same consult service showed that 100% completed all work-up components required to ensure safe and appropriate treatment initiation. The University of Illinois investigated rates of safety monitoring after implementation of a specialty pharmacy service clinical guideline implementation.11 The guidelines were implemented in rheumatology, gastroenterology, dermatology, and transplant clinics for patients receiving biological response modifiers (n = 320).11 Before the guideline was implemented, 31% completed necessary safety tests, while after implementation, 60% completed necessary safety tests (P <.0001).11 Vanderbilt Specialty Pharmacists assisted patients in saving over $5.8 million during fiscal year 2016 through various copay or other patient-assistance programs, which facilitated distribution of more than 14,000 prescriptions that might not have been dispensed otherwise.9 The Cleveland Clinic Specialty Pharmacy found that in a 5-month period, four pharmacists made 547 interventions, with cost savings of over $1.5 million.10
Access and Affordability
In 2021, specialty medications contributed to a total expenditure of $301 billion, accounting for 51% of overall prescription medication expenses.13 This marks a substantial rise since 2012, when specialty medications accounted for 32% of spending.3 The surge in spending can be linked to the rise in new medication approvals, with a 50% increase in orphan medications approved since 2012.3 Per capita spending on specialty medications has experienced nearly a twofold increase, rising from $338 in 2013 to $662 in 2022.3 In contrast, traditional net medicine spending declined by $16 per person within the same time frame.3 Extra costs can be incurred with specialty therapies due to distinctive billing, processing, and handling requirements when compared with nonspecialty medications. An example is a specialty medication given at an infusion center, which would be billed to medical insurance and require specific coding compared with an oral medication that is processed through pharmacy benefits.14
Aside from financial considerations, patients may encounter challenges in accessing specialty therapies due to restrictions tied to preferred network pharmacies. Patients are often required to utilize their plan’s designated specialty pharmacy, which may not be the healthcare system’s specialty pharmacy. This situation could lead to delays in therapy as patients navigate fulfillment restrictions. Furthermore, medication may be transported to the healthcare provider for administration, a process known as white-bagging.2,15 White-bagging entails an external specialty pharmacy delivering medication directly to the administration site, such as healthcare provider’s office or the hospital’s outpatient infusion center. However, some hospitals do not allow white-bagging due to concerns about the source of the therapy and proper storage, which is necessary for compliance with accreditation standards. In such situations, patients may struggle to access care they require. Additionally, brown-bagging involves delivering a specialty medication directly to the patient, who then brings it to the administration site.2,15 Both white-bagging and brown-bagging involve introducing medications into a hospital or healthcare system without direct institutional oversight. While this resembles patients bringing their home medications to the hospital in some ways, it differs because of the strict storage and preparation needs, which may be inadequately monitored if managed by patients or delivered to the infusion center.
Healthcare Provider Collaboration
Collaboration between specialty pharmacies and healthcare providers is paramount to patient-centered care. Due to high costs, special handling, and specific administration, many therapies require prior authorization (PA), in which the healthcare provider obtains preapproval before prescribing a specific drug to ensure coverage according to the terms outlined in the pharmacy benefit plan.16 This process can be a burden on medical staff and can cause therapy delays. As the number of specialty medications continues to rise, prescribers and patients may not always be aware that certain medications require PA before dispensing. Specialty pharmacists may initiate the PA process by contacting the prescriber’s office; however, after contact, PA approval may take days to weeks, leaving patients without medication. Similarly, manufacturer assistance programs provide access to medications for patients who might otherwise go without treatment due to financial constraints.
Time to acquire a PA decreases with use of a specialty pharmacy compared with a physician’s office. Results comparing time for PA decisions between a community-based specialty pharmacy and a dermatology physician’s office showed that the community pharmacy completed 677 PA requests with a mean decision time of 1.9 days, compared with 66 PA requests with a mean decision time of 20.9 days (P <.001) in the physician’s office.17 Additionally, the pharmacy received approval for 96% of the requests, while the physician’s office received approval for 76% of the requests. The pharmacy also demonstrated a mean time to first fill of 6.6 days, compared with 16.2 days with the physician’s office. The introduction of a clinical pharmacist into an infectious disease clinic yielded significant improvement.9 Results showed a 78% decrease in time to medication approval (median 67 days down to 15 days) and a 68% decrease in time to medication initiation (median 82 days down to 26 days).9
Providers and patients rated specialty pharmacy programs highly. A survey conducted in 2012 for patients and healthcare providers to evaluate the satisfaction of a transplantation specialty pharmacy program that showed 84% of patients (n = 290) were satisfied, while 98% would recommend the program to others.18 Of providers (n = 27), 96% believed that the pharmacy improved continuity of care, and 91% reported spending less time on pharmacy-related issues after initiation of the program. A survey in 2021 asked 524 providers about satisfaction involving specialty pharmacies and external specialty pharmacies.19 The score for overall satisfaction (out of 5 points with 5 for “strongly agree” and 1 for “strongly disagree”) with integrated specialty pharmacies was significantly higher than external specialty pharmacies (4.72 ± 0.58 vs. 2.97 ± 1.20, P <.001).
Collaboration between specialty pharmacies and providers ensures that individualized care is accessible to each patient. This collaboration between provider and pharmacist can lead to additional open clinic appointments for an otherwise overscheduled practitioner. Effective communication between providers and pharmacists managing these complex diseases is crucial for medication safety, medication optimization, adherence promotion, monitoring and adjustment, comprehensive care, continuity of care, a safety net for polypharmacy, and most importantly, help to navigate patient-centered care.
Regulatory and Safety Considerations
There are two primary specialty pharmacy accreditation agencies: Accreditation Commission for Health Care and Utilization Review Accreditation Commission.6 Determination of the most suitable accreditation and timing of accreditation are not straightforward decisions. Although accreditation is not legally mandatory for the establishment of specialty services, it is stipulated in a contractual agreement by the primary regional payer within an organization’s service area.2 When there is not a specific mandate, the organization should consider seeking accreditation at an appropriate time after introducing specialty pharmacy services. Accreditors focus on standardization of service, level of patient-care services, and third-party payers, and manufacturers may have more specific accreditation requirements that are contractually required.
Specialty pharmacies are equipped to manage Risk Evaluation and Mitigation Strategies (REMS), as required by the FDA. Specific requirements and key risk messages for REMS are specific to each medication based on potential risk to the patient.20 Certain REMS require certifications to dispense the medication, wherein the pharmacy designates an authorized representative, enrolls the pharmacy, completes required training, and ensures that procedures are in place to train others to comply with REMS requirements. Individual pharmacists may be required to complete training, verify required laboratory monitoring, ensure that patients or prescribers are enrolled in REMS, and provide counseling.
Conclusion
As medicine advances with new breakthroughs and development of complex therapies, there is an increasing demand for specialized pharmacists. Specialized pharmacists demonstrate positive impact on patient care by enhancing health outcomes, promoting patient-centered care, and significantly reducing time required for successful PAs and access to therapies. Research has also revealed substantial cost savings for both healthcare systems and patients when specialty pharmacists oversee pharmaceutical care.
REFERENCES
1. National Association of Specialty Pharmacy. NASP definitions of specialty pharmacy and specialty medications. February 24, 2016. https://a8af0b.p3cdn2.secureserver.net/wp-content/uploads/2020/09/633570_864cb572b8b042909a3f207eaf764d7a.pdf. Accessed October 18, 2023.
2. Cassano A. ASHP Specialty Pharmacy Resource Guide. American Society of Health-System Pharmacists. December 2015. www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/specialty-pharmacy/specialty-pharmacy-resource-guide.ashx. Accessed October 19, 2023.
3. IQVIA Institute for Human Data Science. The global use of medicines 2023. January 2023. www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/the-global-use-of-medicines-2023. Accessed October 24, 2023.
4. Zuckerman AD, Whelchel K, Kozlicki M, et al. Health-system specialty pharmacy role and outcomes: a review of current literature. Am J Health Syst Pharm. 2022;79(21):1906-1918.
5. Myshko D, Wehrwein P. Beyond the big three PBMs. MHE. 2022;32(12):16-20.
6. Fein A. Drug channels: the Specialty Pharmacy Accreditation boom slows: DCI’s exclusive update on the U.S. market. May 18, 2021. www.drugchannels.net/2021/05/the-specialty-pharmacy-accreditation.html. Accessed October 30, 2023.
7. Barnes E, Zhao J, Giumenta A, Johnson M. The effect of an integrated health system specialty pharmacy on HIV antiretroviral therapy adherence, viral suppression, and CD4 count in an outpatient infectious disease clinic. J Manag Care Spec Pharm. 2020;26(2):95-102.
8. Sasnovskaya V, Kumor LM, Stubbings J, Chevalier A. A pharmacist-managed virtual consult service for patients with rheumatologic conditions requiring specialty or infused medications. Am J Health Syst Pharm. 2022;79(1):e41-e49.
9. Bagwell A, Kelley T, Carver A, et al. Advancing patient care through specialty pharmacy services in an academic health system. J Manag Care Spec Pharm. 2017;23(8):815-820.
10. Lankford C, Dura J, Tran A, et al. Effect of clinical pharmacist interventions on cost in an integrated health system specialty pharmacy. J Manag Care Spec Pharm. 2021;27(3):379-384.
11. Hanson RL, Gannon MJ, Khamo N, et al. Improvement in safety monitoring of biologic response modifiers after the implementation of clinical care guidelines by a specialty. J Manag Care Pharm. 2013;19(1):49-67.
12. PQA Adherence Measures. Proportion of days covered. Pharmacy Quality Alliance. April 19, 2022. www.pqaalliance.org/measures-overview. Accessed October 17, 2023.
13. U.S. Department of Health and Human Services. New HHS reports illustrate potential positive impact of Inflation Reduction Act on prescription drug prices. September 30, 2022. www.hhs.gov/about/news/2022/09/30/new-hhs-reports-illustrate-potential-positive-impact-inflation-reduction-act-prescription-drug-prices.html. Accessed October 22, 2023.
14. Centers for Medicare and Medicaid Services. Billing and coding: infusion, injection and hydration services. October 3, 2019. www.cms.gov/medicare-coverage-database/view/article.aspx?articleId=53778&Cntrctr=374&ContrVer=1&CntrctrSelected=374*1&DocType=Active. Accessed October 20, 2023.
15. Pearson C, Schapiro L, Pearson SD. White bagging, brown bagging, and site of service policies: best practices in addressing provider markup in the commercial insurance market. April 19, 2023. https://icer.org/wp-content/uploads/2023/04/ICER-White-Paper-_-White-Bagging-Brown-Bagging-and-Site-of-Service-Policies.pdf. Accessed October 20, 2023.
16. Academy of Managed Care Pharmacy. Prior authorization. July 18, 2019. www.amcp.org/about/managed-care-pharmacy-101/concepts-managed-care-pharmacy/prior-authorization. Accessed October 25, 2023.
17. Hecht B, Frye C, Holland W, et al. Analysis of prior authorization success and timeliness at a community-based specialty care pharmacy. J Am Pharm Assoc. 2021;61(4S):S173-S177.
18. Hlubocky JM, Stuckey LJ, Schuman AD, Stevenson JG. Evaluation of a transplantation specialty pharmacy program. Am J Health Syst Pharm. 2012;69(4):340-347.
19. Anguiano RH, Zuckerman AD, Hall E, et al. Comparison of provider satisfaction with specialty pharmacy services in integrated health-system and external practice models: a multisite survey. Am J Health Syst Pharm. 2021;78(11):962-971.
20. FDA. Roles of different participants in REMS. January 23, 2023. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/roles-different-participants-rems. Accessed October 19, 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





