US Pharm. 2024;49(3):43-47.
ABSTRACT: Lenacapavir (Sunlenca) is the first FDA-approved drug in the novel class of HIV capsid inhibitors for heavily treatment–experienced adults with multidrug-resistant HIV-1 infection after failing their current antiretroviral regimen. It interferes with HIV capsid assembly, virus assembly and release, and nuclear transport. Its unique resistance profile offers promise for patients with multidrug-resistant HIV. Dosing includes initiation with tablets and subcutaneous injection followed by maintenance dosing every 6 months in combination with other antiretroviral therapy. Injection site reactions and nausea are the most common side effects. Cytochrome P4503A, P-glycoprotein, and UGT1A1 interactions are significant.
Lenacapavir is a novel, first-in-class HIV capsid inhibitor that was FDA approved on December 22, 2022, for heavily treatment–experienced adults with multidrug-resistant HIV-1 infection after failing their current antiretroviral (ARV) regimen due to resistance, intolerance, or safety considerations.1
Lenacapavir’s unique mechanism of action and resistance profile offer promise for patients who have run out of ARV treatment options. The drug is administered with an optimized background regimen.2 The starting dosage includes a combination of oral tablets and SC injections, followed by maintenance SC injections every 6 months, offering convenient dosing for patients.
HIV is an infection that poses significant public health challenges worldwide. Globally, the CDC estimates that there are 38 million people living with HIV as of 2020.3 In the United States alone, approximately 1.2 million people are living with HIV, with nearly 37,800 new cases reported in 2019. HIV affects certain populations more significantly, including gay and bisexual men, African Americans, and individuals residing in southern regions of the country.4
HIV attacks the body’s immune system by targeting CD4 T-cells, which are essential to fight off infections and diseases.5 The virus can be transmitted through unprotected sexual intercourse, via contaminated needles or syringes, and from an infected mother to her baby during childbirth or breastfeeding.6 Initial symptoms of HIV infection can resemble flu-like symptoms, including fever, fatigue, sore throat, and swollen lymph nodes.5 These symptoms can vary, and some individuals may not experience any symptoms during the early stages of infection. HIV infection progresses through three stages: the acute stage, clinical latency stage, and acquired immunodeficiency syndrome (AIDS) stage. If not treated, HIV gradually weakens the immune system, leading to opportunistic infections and other serious illnesses. Uncontrolled HIV can progress to AIDS. A person with a healthy immune system typically has a CD4 cell count between 500 cells/mm3 and 1,500 cells/mm3.7 Patients diagnosed with AIDS have a CD4 cell count less than 200 cells/mm3 and/or have acquired one or more opportunistic infections, regardless of CD4 cell count.5
The life cycle of HIV involves multiple steps through which the virus enters and infects CD4 cells and uses the enzymes within the CD4 cell to replicate (see FIGURE 1).8 Understanding the HIV life cycle has been essential to the discovery of targeted therapies to stop viral replication.
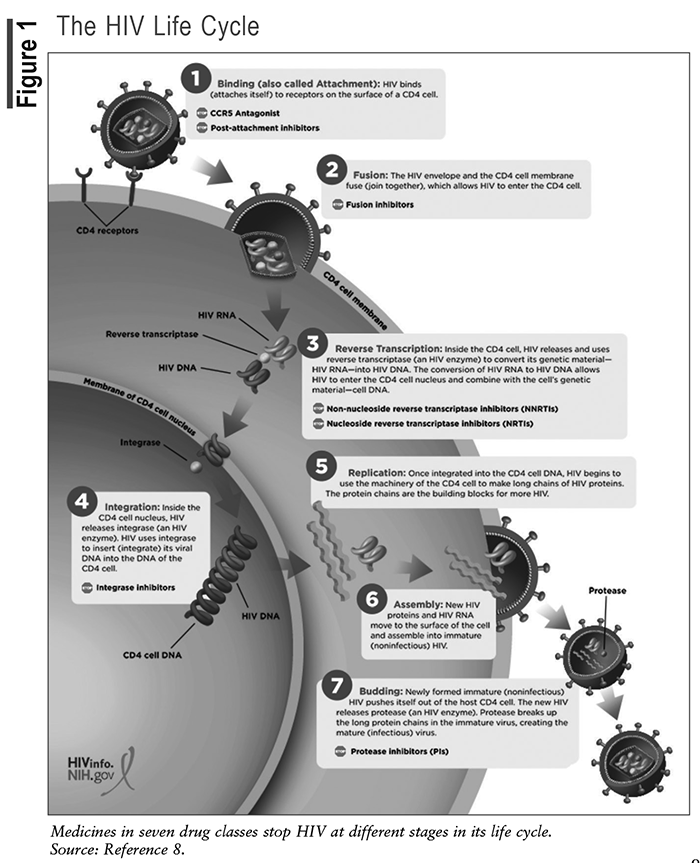
Current ARV Classes and the HIV Life cycle
FDA-approved ARVs are categorized into eight pharmacologic classes, including the nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), fusion inhibitors, CCR5 antagonists, postattachment inhibitors, integrase strand transfer inhibitors (INSTIs), and now the novel class of capsid inhibitors.9
The first step in the HIV life cycle is attachment and entry. HIV attaches to the CD4 receptor and binds to coreceptors, CCR5 or CXCR4, which facilitates entry of the virus into the cell. CCR5 antagonists prevent CD4 cells from expressing their CCR5 coreceptor, which stops HIV from entering the CD4 cell. Postattachment inhibitors bind to the host CD4 receptor and block HIV from binding to the CCR5 and CXCR4 coreceptors.
During the second step in the HIV life cycle, the HIV envelope fuses with the CD4 cell membrane, allowing the viral genetic material to enter the host cell. The fusion inhibitor prevents the membrane of the host CD4 cell from fusing with the HIV envelope. As a result, HIV cannot penetrate the CD4 cell.
The third step in the HIV life cycle involves reverse transcription. Once inside the host cell, the virus converts its RNA genome into DNA using the enzyme reverse transcriptase. This step is crucial for the virus to integrate its genetic material into the host cell’s DNA. NRTIs block the HIV enzyme reverse transcriptase by causing chain termination after being incorporated into the viral DNA. The NNRTIs, on the other hand, bind directly to the enzyme and inhibit transcriptase.
During the fourth step, viral DNA integrates into the host cell’s DNA with the help of the enzyme integrase. This integration allows the virus to remain dormant or replicate along with the host cell. INSTIs prevent HIV from inserting its viral DNA into the DNA of the host CD4 cell by blocking the integrase enzyme. Integrase inhibition stops HIV replication.
The fifth step of the HIV life cycle involves replication and assembly. Integrated viral DNA instructs the host cell to produce new viral RNA and proteins. These components are then assembled into new virus particles. PIs block the enzyme protease and prevent HIV from making new copies of itself to infect other CD4 cells.
The sixth step of the HIV life cycle is assembly. New HIV RNA moves to the surface of the CD4 and assembles into noninfectious HIV.
The seventh part of the HIV life cycle is budding and maturation. The newly formed viral particles bud off the host cell, acquiring an envelope from the host-cell membrane. During this process, the virus undergoes maturation, during which viral protease cleaves the long viral proteins into shorter, functional forms.
The newly released virus infects other CD4 T-cells, perpetuating the cycle of infection. This ongoing infection progressively weakens the immune system over time, leading to the development of AIDS if untreated.
First-Line ARV Therapy
In ARV-naïve patients who have not received treatment for HIV and have no baseline resistance, the guidelines recommend two NRTIs in combination with a third active ARV, including either an INSTI, an NNRTI, or a PI in combination with cobicistat or ritonavir, which are pharmacokinetic enhancers, also known as a boosters.10 Considering the wide variety of treatment options for first-line therapy, choosing a regimen for a specific patient is influenced by factors including efficacy, tolerability, pill burden, dosage frequency, drug-drug interactions, resistance, comorbid conditions, pregnancy, accessibility, and cost.
Lenacapavir Indications and Clinical Profile
Lenacapavir is the first of a new FDA-approved class of drugs called capsid inhibitors to treat HIV-1.2 This drug is indicated for patients who have been heavily treated with ARVs, have developed resistance to many HIV-1 drugs, and are failing their current ARV regimen due to medication resistance, intolerance, or safety considerations.
Pharmacology/Pharmacokinetics
Lenacapavir is a selective inhibitor of HIV-1 capsid function. The drug interferes with three essential steps of the viral life cycle: capsid assembly, virus assembly and release, and nuclear transport.2 Lenacapavir binds to subunits of the HIV capsid protein, which leads to interference in its replication. As a result, new viral particles are improperly shaped and can no longer replicate. Lencapavir’s oral bioavailability is only 6% to 10%, whereas the SC bioavailability is 100%. The Tmax (time it takes to reach maximum plasma concentration) is 4 hours when taken orally and 77 to 84 days when injected SC. The apparent oral volume of distribution is 19,240 L, and the SC apparent volume of distribution is 9,500 to 11,700 L. Lenacapavir’s oral half-life is 10 to 12 days, and its SC half-life is 8 to 12 weeks.
Dosage and Administration
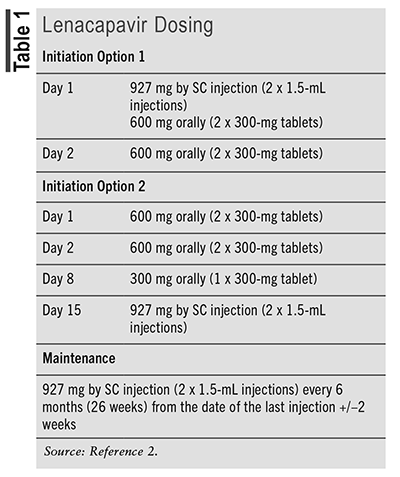
Lenacapavir is commercially available as 300-mg tablets and a 463.5-mg/1.5-mL single-dose vial for injection.2 Lenacapavir is administered as a combination of oral tablets and SC injection for its initiation dose, followed by SC maintenance dosing every 6 months. Two FDA-approved dosing options for lenacapavir are noted in TABLE 1.2 Lenacapavir injection is for administration into the abdomen by a healthcare provider using aseptic technique. The injection is a yellow solution and should not be used if the solution is discolored or contains particulate matter. Once the solution is withdrawn from the vials, the SC injection should be administered as soon as possible into the patient’s abdomen. The injection kit components are for single use only. Two 1.5-mL injections are required for a complete dose. No dosage adjustments are recommended for renal or hepatic insufficiency. Human data on lenacapavir use in pregnancy are lacking. Healthcare providers are encouraged to register patients with the ARV pregnancy registry, which monitors pregnancy outcomes in individuals exposed to lenacapavir during gestation.
Resistance
Drug-resistant mutations have been documented for all ARV classes and can significantly decrease the effectiveness of ARV therapy.11 Several mutations have been identified in the capsid gene that are associated with resistance to capsid inhibitors. The main mutations, M66I and Q67H, are unique to the capsid inhibitors. Mutations that typically develop with current first-line therapy (NRTIs in combination with an NNRTI, PI, or INSTI) are different than those that develop with capsid inhibitors.12 Lenacapavir is fully active against resistant variants with NRTI, NNRTI, PI, and INSTI gene mutations, making it a viable option for heavily pretreated patients with multidrug-resistant HIV-1 infection. For patients who are nonadherent to lenacapavir, an alternative, totally suppressive ARV regimen should be started no later than 28 weeks following the last injection of lenacapavir to reduce the possibility of viral resistance development.2
Adverse Reactions
Injection site reactions (ISRs) (65%) and nausea (4%) are lenacapavir’s most common side effects. ISRs include swelling, discomfort, redness, skin hardening, tiny masses or lumps, and itching.2 Nodules and indurations at the injection site may take longer to go away than the other ISRs. In clinical studies, 30% of nodules and 13% of indurations (in 10% and 1% of patients, respectively) linked with the first injection of lenacapavir had not completely healed after a median follow-up of 553 days. Most nodules and indurations at the injection site were felt but not visible, and their maximum sizes ranged from 1 cm to 4 cm.2
Most patients had mild (Grade 1, 44%) or moderate (Grade 2, 17%) ISRs. Four percent experienced a severe (Grade 3) ISR (erythema, pain, swelling) that resolved within 15 days. The ISRs reported in more than 1% of subjects were swelling (36%), pain (31%), erythema (31%), nodule (25%), induration (15%), pruritus (6%), extravasation (3%), and mass (3%). ISRs reported in 1% of subjects included discomfort, hematoma, edema, and ulcer.2
Drug-Drug Interactions
Lenacapavir is a moderate inhibitor of cytochrome P450 3A (CYP3A) and a substrate of P-glycoprotein (P-gp) and UGT1A1.2,13 Lenacapavir plasma concentrations may be lowered by medications that are strong or moderate CYP3A inducers. Coadministration of these medications is not advised. Strong CYP3A inhibitors, coupled P-gp, and UGT1A1 inhibitors should not be taken with lenacapavir because they may significantly increase the drug’s plasma concentration. Lenacapavir may enhance the exposure of medications largely metabolized by CYP3A that are initiated within 9 months after the last SC dosage of lenacapavir because of the long half-life.
Cost
The average wholesale price for the initiation dose options ranges from $3,900 to $4,875, and each 6-month SC dose is $23,400.14 Actual costs for patients will depend on their insurance coverage, copays, and financial assistance from the manufacturer.
Role of the Pharmacist
The unique dosing and administration associated with lenacapavir are important counseling points. Pharmacists should counsel patients to take lenacapavir tablets only during the initiation phase and with or without food.2 SC maintenance dosing will be administered by a healthcare provider every 6 months (24 weeks). It is important for patients to keep appointments with their healthcare provider to receive maintenance injections on time. If more than 28 weeks have elapsed since the last injection and it is clinically appropriate to continue lenacapavir, patients will need to restart initiation therapy with the oral tablets and SC injections from Day 1, using either initiation dosing option 1 or 2 (TABLE 1).2 Pharmacists should counsel patients that lenacapavir will stay in their body for up to 9 months after their last dose. If patients discontinue lenacapavir, they should inform their healthcare provider before starting, stopping, or changing the dose of any other drug since multiple drug interactions can occur with lenacapavir. Herbal products can also interact with ARVs and should be avoided. Patients also need to be counseled that lenacapavir will be administered in combination with other antiretrovirals based on their prior ARV use and presence of resistance mutations. While lenacapavir maintenance dosing is only administered every 6 months, they may still be taking other ARVs daily. Adherence to all ARVs is essential to achieve viral suppression, preserve the immune system, and prevent the further development of resistance.
Summary
Lenacapavir is a pioneer in the novel class of HIV capsid inhibitors. It is highly potent and long acting, with a unique resistance profile, making it a viable treatment option for heavily pretreated patients who have developed resistant mutations to other ARVs. The drug is initially administered with a combination of tablets and SC injection followed by convenient SC dosing every 6 months. Lenacapavir’s most common side effects are ISRs and nausea. Lenacapavir has the potential for multiple and significant drug interactions through CYP3A, P-gp, and UGT1A1. Lenacapavir represents the first in a new class of drugs to target three distinct areas of the HIV life cycle and offers promise for people living with HIV.
REFERENCES
1. FDA. FDA approves new HIV drug for adults with limited treatment options. December 22, 2022. www.fda.gov/news-events/press-announcements/fda-approves-new-hiv-drug-adults-limited-treatment-options. Accessed August 4, 2023.
2. Sunlenca (lenacapavir) product information. www.gilead.com/-/media/files/pdfs/medicines/hiv/sunlenca/sunlenca_pi.pdf. Accessed August 4, 2023.
3. CDC. HIV: statistics overview. August 10, 2022. www.cdc.gov/hiv/statistics/overview/index.html#:~:text=Worldwide%2C%20there%20were%20about%201.5,AIDS%2Drelated%20illnesses%20in%202020. Accessed August 4, 2023.
4. CDC. HIV: basic statistics. May 22, 2023. www.cdc.gov/hiv/basics/statistics.html. Accessed August 4, 2023.
5. CDC. About HIV/AIDS. June 30, 2022. www.cdc.gov/hiv/basics/whatishiv.html. Accessed August 4, 2023.
6. CDC. Ways HIV can be transmitted. March 4, 2022. www.cdc.gov/hiv/basics/hiv-transmission/ways-people-get-hiv.html. Accessed August 4, 2023.
7. Battistini Garcia SA, Guzman N. Acquired immune deficiency syndrome CD4+ count. StatPearls Publishing [Internet]. August 8, 2022. www.ncbi.nlm.nih.gov/books/NBK513289/. Accessed August 4, 2023.
8. NIH. The HIV Life Cycle. HIVInfo.NIH.gov. August 4, 2021. https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-life-cycle. Accessed August 4, 2023.
9. HIV/AIDS Glossary. Drug class. ClinicalinfoHIVgov. https://clinicalinfo.hiv.gov/en/glossary/drug-class. Accessed August 4, 2023.
10. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv. Accessed September 19, 2023.
11. Wensing A, Calvez C, Ceccherini-Silberstein F, et al. 2022 Update of the drug resistance mutations in HIV-1. IAS-USA. Top Antiv Med. 2022;30(4):559-574.
12. Sunlenca. www.sunlencahcp.com/. Accessed August 4, 2023.
13. NIH. SUNLENCA-lenacapavir sodium tablet, film coated; SUNLENCA-lenacapavir sodium kit. DailyMed. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e5652804-29c4-40d7-aeb2-0142ed2a7b5b. Accessed August 4, 2023.
14. Red Book. Micromedex (electronic version), IBM Watson Health information. www.micromedexsolutions.com/. Accessed August 4, 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





