US Pharm. 2019;44(7):HS-8-HS-16.
ABSTRACT: Inhalers used in the treatment of chronic obstructive pulmonary disorder (COPD) come in a variety of novel mono-, dual-, and triple-therapies. These inhalers may contain short-acting beta2 agonists, long-acting beta2 agonists, short-acting muscarinic antagonists, long-acting muscarinic antagonists, or inhaled corticosteroids. In recent years, novel inhalers have entered the market in a variety of delivery devices, active ingredients, and costs. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines classify a patient’s COPD group and provide first-line therapy options. Improper inhaler technique and cost may pose a barrier to medication adherence. Inhaler selection should be individualized based on patients’ GOLD COPD classification, preference, ease of inhaler use, and cost.
Chronic obstructive pulmonary disorder (COPD) develops over time as the small airways become inflamed due to the inhalation of cigarette smoke or other noxious particles. The chronic inflammatory response may induce parenchymal tissue destruction resulting in emphysema, the disruption of normal repair and defense mechanisms resulting in small airway fibrosis. Generally, the inflammatory and structural changes of the small airways increase with disease severity.
Patients with COPD typically present with progressive shortness of breath, a chronic cough or recurrent wheeze, and chronic sputum production. Patients’ airflow limitation with a post-bronchodilator forced expiratory volume/forced vital capacity (FEV1/FVC) <0.7 is further classified based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines as either GOLD 1 (mild), GOLD 2 (moderate), GOLD 3 (severe), or GOLD 4 (very severe). Patients’ symptom burden and risk of exacerbation are classified into GOLD groups A through D; this is used to guide patients’ therapy. Classification of airflow limitation (grades 1-4) and symptom burden with exacerbation risk (groups A-D) is patient-specific and can occur in a variety of combinations.
Pharmacologic therapy for COPD is used to decrease symptoms, reduce the frequency and severity of exacerbations, and improve exercise intolerance. Common classes of medications used in treatment of COPD include beta2 agonists, antimuscarinics, inhaled corticosteroids (ICS), and combination therapy. Identification and reduction of exposure to risk factors, such as cigarette smoke, air pollutants, and occupational fumes, are also important in treatment and prevention of COPD. This review will summarize the updated 2019 GOLD recommendations on managing COPD, along with evidence and cost information on various inhalers.1
Diagnosis, Management, and Prevention of COPD
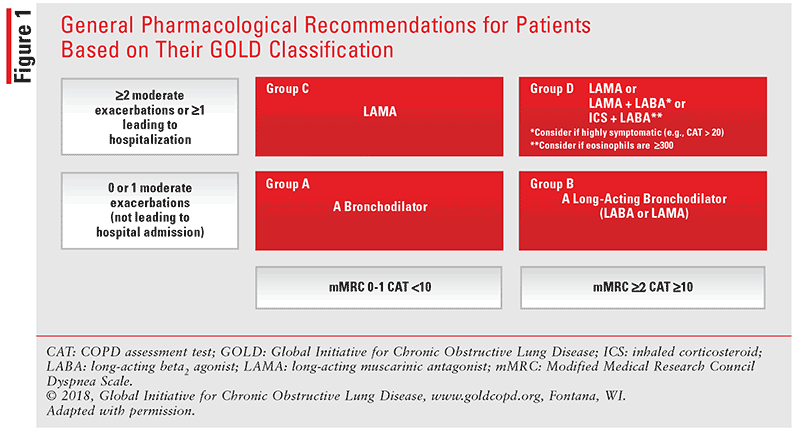
According to the GOLD 2019 Global Strategy for the Diagnosis, Management, and Prevention of COPD guideline update, first-line pharmacologic therapy depends on the patient’s GOLD classification (FIGURE 1.) Short-acting bronchodilators (short-acting muscarinic antagonist [SAMA] or short-acting inhaled beta2 agonist [SABA]) should be prescribed to all patients for immediate symptom relief, regardless of their GOLD classification.1
For Group A patients, a short- or long-acting bronchodilator (long-acting muscarinic antagonist [LAMA] or long-acting beta2 agonist [LABA]) is recommended based on their effects on patients’ breathlessness.
For patients classified in Group C, initial therapy should consist of a long-acting bronchodilator; LAMAs are superior to LABAs regarding COPD exacerbation.
For Group B patients, the guidelines do not recommend one class of long-acting bronchodilator over another for initial symptoms; initial therapy with two long-acting bronchodilators may be considered in patients who are experiencing severe breathlessness on monotherapy.
In Group D, a LAMA/LABA combination can be chosen as initial treatment in patients experiencing more severe symptoms, such as greater dyspnea and/or exercise intolerance. The 2019 guideline update recommends a LABA/ICS combination for initial treatment in patients with an eosinophil count greater than 300 cells/µL or those with a history of asthma and COPD. Patients who develop exacerbations while on a LAMA/LABA may be escalated to a LABA/LAMA/ICS, including the once-daily inhaler fluticasone furoate/umeclidinium/vilanterol (Trelegy Ellipta).
Preventive measures recommended by the 2019 GOLD guidelines include vaccinations and smoking cessation. The yearly influenza vaccine and the PPSV23 and PCV13 pneumococcal vaccines are recommended in all patients with COPD.2 PPSV23 is recommended for patients aged 19 to 64 years, and PCV13 is recommended for patients aged 65 years and older, administered at least 1 year after PPSV23. Smoking cessation has the greatest ability to influence COPD disease progression.3 The guidelines recommend brief interventions, such as asking about tobacco use; advising the user to quit; assessing willingness to quit; assisting in quitting; and arranging follow-up contact with the patient. OTC quit aids include nicotine gum, lozenges, and patches.
Clinical Trials in COPD Management
The SUMMIT study by Calverley and colleagues compared fluticasone furoate monotherapy (Arnuity Ellipta), fluticasone furoate with vilanterol (Breo Ellipta) and vilanterol monotherapy and their rates of FEV1 decline.4 The purpose of the study was to assess whether drug treatment could modify loss of lung function in patients with GOLD grade 2, or moderate COPD. Spirometry was measured every 12 weeks as part of a randomized, placebo-controlled trial of 16,485 patients with GOLD grade 2 COPD. Results indicated a decline in FEV1 of 38 mL/y in those using fluticasone furoate in combination with vilanterol or as monotherapy as compared with placebo (-46 mL/y, P <.03) and vilanterol monotherapy (-47 mL/y, P <.005). FEV1 decline was found to be greater in current smokers, those with lower BMI, males, and patients with established cardiovascular disease. In patients with moderate COPD and heightened cardiovascular risk, fluticasone furoate alone or in combination with vilanterol significantly reduced the rate of FEV1 decline.
The SPARK study by Wedzicha and and colleagues evaluated the effect of dual, long-acting bronchodilator therapy on exacerbations in patients with GOLD grades 3-4, or severe and very severe COPD, with one or more exacerbations in the past year.5 In this parallel group study, 2,224 patients were randomly assigned to once-daily QVA149 (fixed-dose combination of indacaterol/glycopyrronium 110/50), glycopyrronium 50 µg, or tiotropium 18 µg. Patients receiving once-daily treatment with QVA149 or glycopyrronium were both double-blinded, while the once-daily tiotropium treatment group was open-label. QVA149 resulted in a statistically significant decrease in mild (15%, P = .0072) and moderate-to-severe (12%, P = .038) exacerbations compared with the glycopyrronium treatment group. Compared to tiotropium, there was a statistically significant decrease in mild (16%, P = .0052) exacerbations in the QVA149 treatment group. There were no statistically significant differences between treatment groups with regard to adverse medication events such as bacterial upper-respiratory tract infection, nasopharyngitis, and viral upper-respiratory tract infection. Overall, the dual bronchodilator QVA149 was superior in preventing moderate-to-severe COPD exacerbations as compared with glycopyrronium and tiotropium. These results indicate a potential benefit in dual bronchodilation as a treatment option for patients with severe and very severe COPD.
The IMPACT trial by Lipson and colleagues aimed to assess the efficacy of a novel triple-therapy inhaler, fluticasone furoate/umeclidinium/vilanterol (Trelegy Ellipta), versus traditional fluticasone furoate/vilanterol (Breo Ellipta) or umeclidinium/vilanterol (Anoro Ellipta) therapy.6 In the double-blind, parallel-group, randomized controlled trial, 10,355 patients were studied in 37 countries from June 2014 to July 2017. The IMPACT trial aimed to assess the rate of COPD exacerbations in patients with GOLD grades 2-4 COPD during treatment with each therapy over 52-week periods. A moderate exacerbation was defined as one that required treatment with oral/systemic corticosteroids and/or antibiotics that did not result in hospitalization, whereas a severe exacerbation would result in hospitalization or death. Results demonstrated an incidence of moderate or severe exacerbations as 1.07 and 1.21 per year in the fluticasone furoate/vilanterol and umeclidinium/vilanterol groups, respectively, as compared with 0.92 per year in the fluticasone furoate/umeclidinium/vilanterol group (P <.001). In the average COPD population, yearly exacerbations are between two and three.7 Common adverse events (1%-10% incidence) reported for the fluticasone furoate/umeclidinium/vilanterol group were pneumonia, lower-respiratory tract infection, cardiac arrhythmia, and anticholinergic effects such as dry mouth or confusion. The authors concluded that use of fluticasone furoate/umeclidinium/vilanterol resulted in a lower rate of moderate or severe COPD exacerbations versus the traditional fluticasone furoate/vilanterol and umeclidinium/vilanterol therapy. Fluticasone furoate/umeclidinium/vilanterol was also shown to reduce the rate of hospitalizations when compared to umeclidinium/vilanterol therapy.6
Adverse Effects of COPD Inhalers
Beta2 agonists (SABAs, LABAs) can produce sinus tachycardia and precipitate cardiac-rhythm disturbances in susceptible patients. Hypokalemia can occur, especially when beta2 agonists are combined with thiazide diuretics, as can increased oxygen consumption in patients with heart failure, but these effects decrease over time.8,9
Inhaled antimuscarinics (SAMAs, LAMAs) are poorly absorbed, which limits systemic side effects. The main side effect of inhaled antimuscarinics includes dry mouth. Some patients using ipratropium reported a bitter, metallic taste following use. There have also been reports of a small increase in cardiovascular events in COPD patients treated with ipratropium.10 However, in a large, long-term clinical trial in COPD patients, tiotropium added to standard therapies had no effect on cardiovascular risk.11
Inhaled corticosteroids such as fluticasone and mometasone are also associated with superficial adverse drug events such as oral candidiasis (thrush), hoarse voice, skin bruising, and pneumonia.12 To mitigate these risks, patients should “swish and spit” after administration.
Common adverse events of the novel triple combination inhaler fluticasone furoate/umeclidinium/vilanterol include cough, headache, backache, diarrhea, and altered sense of taste.13 It is important to note that fluticasone furoate/umeclidinium/vilanterol has a higher incidence of pneumonia compared with LAMA/LABA combinations such as umeclidinium/vilanterol. There are no significant differences for the risk of pneumonia between fluticasone furoate/umeclidinium/vilanterol and LABA/ICS inhalers.6
Proper Inhaler Techniques
Reviewing inhaler technique is recommended at initiation and follow-up. The novel inhalers on the market come in a variety of delivery devices such as Ellipta, Pressair, Respimat, and Neohaler.
Ellipta: Umeclidinium (Incruse Ellipta) and umeclidinium/vilanterol (Anoro Ellipta) are formulated as Ellipta devices containing an inhalation powder. To use an Ellipta inhaler: Slide the cover down until a click is heard, breathe out gently (away from inhaler), put the mouthpiece in the mouth and close the lips, to form a good seal (but do not cover vents), breathe in steadily and deeply, hold the breath for 5 seconds, breathe out gently, and slide the cover upward as far as it will go to cover the mouthpiece.14
Pressair: Aclidinium bromide (Tudorza Pressair) is formulated as a Pressair device containing an inhalation powder. To use a Pressair inhaler: Remove the protective cap by gently squeezing the arrows on the side of each cap, hold the inhaler with the mouthpiece facing you with the green button facing up, press the green button down and release before placing mouthpiece in mouth, assure the control window has changed from red to green, breathe out gently (away from inhaler), put the mouthpiece between the lips, and breathe in quickly and deeply.15
Respimat: Olodaterol (Striverdi Respimat) is formulated as a Respimat device containing an inhalation spray. To use a Respimat: After initial priming, hold inhaler upright and turn base in direction of arrows on the label until it clicks (half of a turn), open cap until it snaps fully open, breathe out (away from inhaler), put mouthpiece between the teeth and close the lips to form a good seal (but do not cover vents), breathe in slowly and deeply through the mouth while pressing down on the dose button, hold the breath for 5 seconds and remove the inhaler from the mouth, breathe out gently, and replace the cap.16
Neohaler: Glycopyrronium/indacaterol (Utibron Neohaler) is formulated as a Neohaler dry-powder device. To use a Neohaler inhaler: Remove the cap, tilt the mouthpiece to open the inhaler, remove one capsule from the blister card, place the capsule into the capsule chamber, close the mouthpiece fully, hold the inhaler with the mouthpiece facing up and press both piercing buttons at the same time, release buttons, breathe out gently (away from inhaler), place the mouthpiece in the mouth, breathe in steadily and deeply, hold the breath for 5 seconds, breathe out gently, and remove the capsule from the capsule chamber.17
Pictorial representation of how to operate these devices can be found in the inhalers’ package inserts.
Comparison of Inhalers
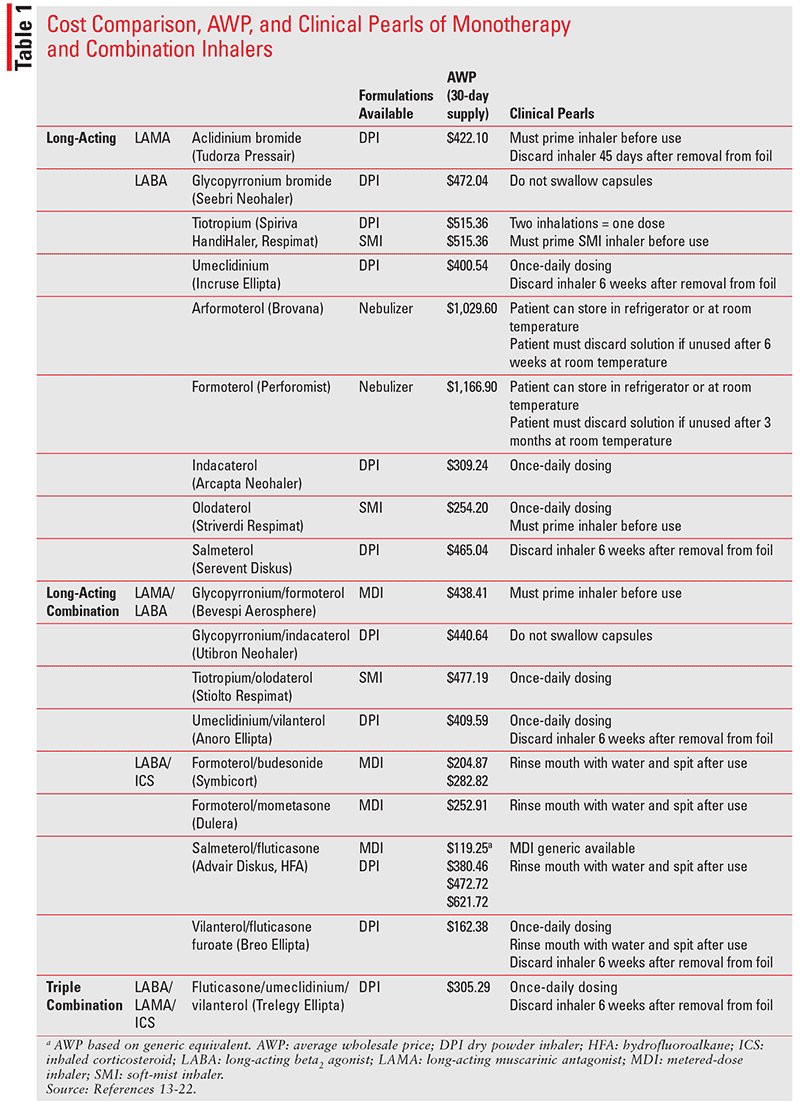
TABLE 1 summarizes the average wholesale prices of different inhalers on the U.S. market. Umeclidinium (Incruse Ellipta) is a LAMA monotherapy inhaler that provides a once-daily dosing option for patients as compared with aclidinium bromide (Tudorza Pressair), which is dosed twice daily.14,15 With regard to LABA monotherapy inhalers, olodaterol (Striverdi Respimat) provides a once-daily dosing option for patients and is less expensive among other LABA monotherapies.16 Fluticasone furoate/vilanterol (Breo Ellipta) is a once-daily LABA/ICS combination inhaler.18 Note that fluticasone furoate/vilanterol received a new warning in January 2019 for both increased intraocular pressure and risk of glaucoma as well as hyperglycemia, which warrants additional monitoring in those with a history of type 2 diabetes mellitus.18
The 2019 GOLD guidelines include the once-daily LABA/LAMA/ICS combination inhaler fluticasone/umeclidinium/vilanterol. In addition to its appearance in the 2019 GOLD guidelines, a new warning was placed in the fluticasone/umeclidinium/vilanterol’s package insert for patients with narrow-angle glaucoma. Glaucoma, increased intraocular pressure, and cataracts have been reported with use of fluticasone/umeclidinium/vilanterol. Patients should report to a healthcare provider any eye pain or discomfort, blurred vision, or visual halos while using fluticasone/umeclidinium/vilanterol.13 These monotherapy and combination inhalers were introduced to the market within the past decade and vary in their costs and device technique.
Conclusion
There are a variety of inhalers for the treatment of COPD such as SABA, LABA, SAMA, LAMA, ICS, and combinations of these. First-line therapies are dependent upon a patient’s GOLD classification, as well as other patient-specific factors such as cost and type of inhaler. Also included in the 2019 GOLD update is a triple combination-therapy inhaler, fluticasone/umeclidinium/vilanterol (Trelegy Ellipta), which provides a once-daily option for patients with more severe COPD. There are several other monotherapy and combination inhalers that provide the option for once-daily dosing, which may be favorable for patients. Novel inhalers released within the past decade vary in cost and dosing frequency. These provide patients with more options to treat their COPD based on individual preferences. Inhalers used in the treatment of COPD are generally well tolerated. It is important for the pharmacist to assess inhaler technique and understand how each inhaler is used with each follow-up or encounter with patients. Other strategies to manage COPD include the pneumococcal vaccine, yearly influenza vaccine, and smoking cessation. COPD inhaler therapy should be individualized based on cost, patients’ preference, and their COPD classification.
REFERENCES
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2019 report. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed March 22, 2019.
2. CDC. Recommended adult immunization schedule 2019. http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed March 22, 2019.
3. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville, Maryland: U.S. Department of Health and Human Services. Public Health Service; May 2008. www.ncbi.nlm.nih.gov/books/NBK63952. Accessed March 22, 2019.
4. Calverley PMA, Anderson JA, Brook RD, et al. Fluticasone furoate, vilanterol, and lung function decline in patients with moderate chronic obstructive pulmonary disease and heightened cardiovascular risk. Am J Respir Crit Care Med. 2018;197(1):47-55.
5. Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199-209.
6. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2019;378(18):1671-1680.
7. Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418-1422.
8. Kohansal R, Martinez-Camblor P, Agusti A, et al. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180(1):3-10.
9. Raad D, Gaddam S, Schunemann HJ, et al. Effects of water-pipe smoking on lung function: a systematic review and meta-analysis. Chest. 2011;139(4):764-774.
10. Liu S, Zhou Y, Liu S, et al. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: results from a cross-sectional study in China. Thorax. 2017;72(9):788-795.
11. Gauderman WJ, Avol E, Gilliland F, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351(11):1057-1067.
12. Polosukhin VV, Richmond BW, Du RH, et al. Secretory IgA deficiency in individual small airways Is associated with persistent inflammation and remodeling. Am J Respir Crit Care Med. 2017;195(8):1010-1021.
13. Trelegy Ellipta (fluticasone/umeclidinium/vilanterol) package insert. Research Triangle Park, NC: GlaxoSmithKline; 2018.
14. Incruse Ellipta (umeclidinium) package insert. Research Triangle Park, NC: GlaxoSmithKline; 2013.
15. Tudorza Pressair (aclidinium bromide) package insert. St. Louis, MO: Almirall; 2012.
16. Striverdi Respimat (olodaterol) package insert. Ridgefield, CT: Boehringer Ingelheim; 2014.
17. Utibron Neohaler (glycopyrronium/indacaterol) package insert. East Hanover, NJ: Novartis; 2015.
18. Breo Ellipta (vilanterol/fluticasone furoate) package insert. Research Triangle Park, NC: GlaxoSmithKline; 2013.
19. Anoro Ellipta (umeclidinium/vilanterol) package insert. Research Triangle Park, NC: GlaxoSmithKline; 2013.
20. Stiolto Respimat (tiotropium/olodaterol) package insert. Ridgefield, CT: Boehringer Ingelheim; 2015.
21. Bevespi Aerosphere Glycopyrronium/formoterol package insert. Wilmington, DE: AstraZeneca; 2016.
22. Red Book Online [database on Internet]. Greenwood Village (CO): Truven Health Analytics. www.micromedexsolutions.com. Accessed March 24, 2019.
To comment on this article, /contact rdavidson@uspharmacist.com.