US Pharm. 2012;37(11):HS16-HS19.
ABSTRACT: The vast increase in the number of new psychopharmacologic agents has made more therapeutic options available, but it has also complicated patient treatment. Combination therapy used in psychiatric practice makes drug interactions more likely and increases the risk of adverse outcomes to patients. The amount of information on drug-drug interactions is overwhelming, so health systems have implemented the use of computer software to assist in the detection of these problematic combinations. While these systems offer necessary support, the pharmacist’s expertise in triaging these alerts and communicating the relevance to the prescriber is essential. This review will provide an essential refresher on psychiatric drug interactions for institutional practitioners as well as offering suggestions to optimize patient safety when patients are on medication regimens that include psychiatric drugs.
The vast increase in the number of new psychopharmacologic agents over the last 20 years has made more therapeutic options available, but it also has made treating patients more complicated. Prescribing practices, which include the concurrent administration of a variety of psychotropic drugs, have made the risk of drug-drug interactions more likely. Drug interactions are known to play a significant role in the incidence of adverse drug reactions (ADRs) both in the community and in hospitals. Reducing ADRs is a critical element in providing safe medication use for hospitalized patients. According to a recently published study, psychiatric medications can account for up to 50% of the ADRs for hospitalized patients with mental illness, many of which can be attributed to drug-drug interactions.1 ADRs resulting from drug-drug interactions leading to hospitalization are often preventable. It has been estimated that 26% of ADRs requiring hospital admissions may be due to drug-drug interactions.2
Even ADRs that are deemed to be “not severe” can have significant impact on patients with a psychiatric illness, as a growing body of evidence suggests a strong relationship between drug-drug interactions, treatment failures, and higher health care costs due to avoidable medical complications.3 While only a few of the possible drug interactions may be clinically relevant, the practitioner still must consider critical factors associated with drug-drug interactions. Such factors include the potency and concentration of the drugs involved, the therapeutic index balanced between efficacy and toxicity, the presence of active metabolites, and the extent of the metabolism of the substrate drug.4
Pharmacokinetic Drug Interactions
Absorption: Psychiatric drug interactions resulting from impaired absorption are similar to those seen with medical medications. For example, psychiatric patients on certain medication regimens, such as the atypical antipsychotic clozapine, can develop significant constipation, which often requires additional medication to resolve. Bulk laxatives such as psyllium, magnesium-based antacids, and lactulose products may reduce the absorption of other drugs if administered at the same time.
There has been increasing focus on the role of the drug transporter P-glycoprotein (Pgp) in drug absorption into the brain. While the tissue distribution of Pgp influences the effect of psychotropics and the interaction potential for drugs such as risperidone, nortriptyline, and citalopram at the interface between the blood and central nervous system (CNS), Pgp is also found in other areas of the body such as the intestines, which are a major site for drug absorption into the body.5 The Pgps found in the gut have not been as extensively studied; however, it is well known that the expression of Pgp in other tissues can be induced and inhibited by other drugs. It is thought that some interactions, mainly seen with the antiepileptic drugs (AEDs), previously assumed to be a result of CYP450 alterations, instead may actually be mediated by the modulation of the Pgp activity at the point of drug absorption or distribution.3,6 In general, chelation is not as much an issue for antipsychotics; however, antacids containing divalent cations (such as calcium and/or magnesium) and sucralfate may impair the absorption of phenytoin.7
Metabolism: Many key enzyme pathways are involved in psychiatric drug interactions, the most prominent being the CYP450 system. Unlike inhibition that occurs immediately when drug binds to substrate, inducers responsible for increasing hepatic metabolism can require days or weeks to produce the most significant effect and therefore may not substantially impact treatment decisions in an acute care environment. Examples of some classic inducers are carbamazepine, phenytoin, primidone, and phenobarbital.3
When considering acute care hospitalization, one metabolic consequence of induction that must be considered upon admission is the impact of mandated smoking cessation on the patient. Medications that are metabolically induced by the isoenzyme CYP1A2, such as clozapine, can see up to a 50% serum concentration increase when the liver enzymes return to baseline activity without the influence of the polycyclic aromatic hydrocarbons (PAH) in cigarette smoke.8,9 Though the PAH impact varies with the number of cigarettes smoked, a patient who smokes at least a pack per day may need a proactive dose reduction of up to 10% per day for 5 days to accommodate this metabolic consequence of drugs with a narrow therapeutic range.10 Increased serum concentrations of some medications (e.g., olanzapine) result only in increased bothersome side effects such as drowsiness, but others (e.g., clozapine) can lead to much more serious adverse effects, including seizures.
With few exceptions, psychiatric drugs are lipophilic agents that are extensively metabolized in phase I oxidative metabolism. Most of the new psychotropic agents either are metabolized by or inhibit to varying degrees one or more of the CYP enzyme systems (TABLE 1).4
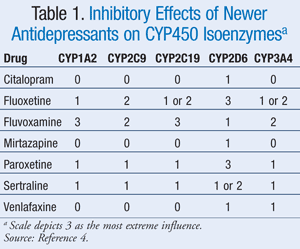
Though phase II metabolism generally does not contribute as significantly as phase I metabolism for psychiatric drugs, the most well-known phase II enzyme family is the uridine 5'-diphosphate glucuronosyltransferases (UGTs). Like the CYP450 enzyme system, the UGTs have substrates, inhibitors and inducers, and numbering schemes. Some of the well-known interactions within the phase II system occur with but are not limited to lamotrigine, olanzapine, and many of the narcotic analgesics.3
Psychiatric drug interactions that result in serum concentration changes are generally most relevant for drugs with a narrow therapeutic index such as lithium and clozapine, where increases or decreases play a role in worsening clinical condition or increasing the risk of serious adverse effects. However, for many of the psychiatric agents, an increase in serum concentration represents a predictably increased degree of drowsiness and dizziness and thus can be managed safely with appropriate precautions.
Distribution: Protein-binding interactions can occur when two or more highly protein-bound drugs compete for a limited number of binding sites on plasma proteins.11 The risk for protein-binding interactions occurs as the unbound free fraction of the competing drug increases and becomes more available for metabolism. This is more common for the mood-stabilizing AEDs, including phenytoin, valproic acid, diazepam, and tiagabine, as well as for antipsychotics, including clozapine, risperidone, olanzapine, and ziprasidone.7,11 However, these medications are often present in such small quantities in the blood that their contribution to displacement of other highly protein-bound drugs does not generally result in clinically relevant displacement and subsequently significantly altered therapeutic actions.7 Typically in the young and physically healthy, protein-displacement drug interactions are not usually significant since there is a compensatory drug clearance that results from the larger unbound fraction of drug available for metabolism.11 The theorized risk of plasma protein–displacement interactions can translate into an unnecessary worry for the practitioner who may not be fully aware of the actual clinical impact on his or her patient.
Elimination: Psychiatric drug interactions that result in altered elimination are rare; however, the few medications that are included in this category, such as lithium, can quickly reach toxic serum concentrations when other drugs are added without consideration of risk. Medications administered in acute care such as nonsteroidal anti-inflammatory drugs (NSAIDs), angiotensin-converting enzyme (ACE) inhibitors, or angiotensin II receptor blockers (ARBs) should be used cautiously, if at all, in patients already receiving lithium.11 Indomethacin and piroxicam have been reported to significantly increase steady-state plasma lithium concentrations due to a proposed alteration of prostaglandin involvement in the renal clearance and excretion of lithium. An increase in lithium levels can develop over 5 to 10 days after adding an NSAID, and levels can return to baseline serum concentrations within 7 days of stopping the NSAID. In the case of ACE inhibitors and ARBs, it has been suggested that these agents decrease lithium clearance as a result of sodium depletion, which leads to increased renal tubular reabsorption of lithium.12
Pharmacodynamic Drug Interactions
Pharmacodynamic drug-drug interactions occur when drugs act at the same or interrelated receptor sites, resulting in additive, synergistic, or antagonistic effects of each drug at the target receptor. Pharmacodynamic interactions, which result in a potentiation of the pharmacologic effects at the receptor, can be very important clinically. Most pharmaco-dynamic interactions are fairly straightforward and predictable if the practitioner has a basic understanding of the drug mechanism of action and receptor effects; therefore, the interactions can be anticipated, avoided, or managed when the combination is medically required.3
Whether the unintended effects of simultaneous drug therapy are due to an additive or cumulative effect of combined drug therapy, such exaggerated pharmacologic effects require practitioners engaged in psychiatric medication management to exercise great caution and be prepared to intervene quickly. Some examples of psychiatric pharmaco-dynamic drug interactions are discussed below.
Anticholinergic Intoxication: The synergistic anticholinergic effect of drugs such as tricyclic antidepressants administered concurrently with antiparkinsonian agents can increase the anticholinergic effects of antipsychotics such as clozapine, olanzapine, and quetiapine, leading to dry mouth, blurred vision, and possibly delirium.10 Amitriptyline taken concurrently with benztropine can produce pronounced constipation, heat stroke, urinary retention, and other shared side effects with exaggerated intensity.3
Serotonin Syndrome: The neurotransmitter serotonin is involved in multiple bodily processes including aggression, pain, appetite, depression, and migraine. Potentially fatal, serotonin syndrome is caused by an increase in the amount of serotonin action in the CNS. Research has determined that overstimulation of the 5-HT2A receptor appears to be substantially responsible for this reaction. The serotonin (5-HT1A) receptor also contributes through a pharmacodynamic interaction in which increased synaptic concentration of a serotonin agonist saturates all receptor sites, thus magnifying the sum of serotonergic action. Drug categories that should be considered in this possible interaction include antidepressants, opioids, CNS stimulants, 5-HT1 agonists (triptans), dextromethorphan, and certain herbal products available OTC (e.g., St. John’s wort).13
QTc Prolongation and Torsades de Pointes (TdP): A pharmacodynamic drug interaction may be the result of combining two or more drugs with established risk of prolongation of QTc interval such as chlorpromazine and haloperidol, placing the patient at a greater risk for adverse effects such as malignant arrhythmias. Uncertainty remains regarding the specific relationship between the extent of QT prolongation and the risk of life-threatening arrhythmias with each individual drug. QT interval prolongation of at least 500 milliseconds (ms) generally has been shown to correlate with an increased risk of TdP. In contrast, there is no established threshold below which prolongation of the QT interval is considered free of risk.10,14-16
Blood Dyscrasias: Almost all classes of psychotropic agents have been reported to cause blood dyscrasias. Leukopenia, neutropenia, thrombocytopenia, eosinophilia, anemia, agranulocytosis, and altered platelet function are some of the hematologic side effects that may be encountered with psychiatric medication therapy. Clozapine is well known as a drug that causes dyscrasias; however, many other agents, including olanzapine, antidepressants, mood-stabilizing AEDs (e.g., divalproex), and other atypical antipsychotics can cause similar problems.17
Other Issues: Coadministration of many antipsychotic agents (such as olanzapine concomitantly administered with conventional agents such as haloperidol or with atypical agents such as lurasidone) may increase the risk of adverse effects such as neuroleptic malignant syndrome (NMS) or seizures and/or can result in the addition of other more common adverse effects such as drowsiness, dizziness, orthostatic hypotension, anticholinergic effects, and extrapyramidal symptoms.11
Drug Interaction Alerts
The amount of information on drug-drug interactions far exceeds the limits of human memory and recall. The recognition of this limitation has led to the development and use of sophisticated computer software that predicts a portion of the almost limitless permutations of drug-drug interactions. Though helpful in offering the first line of defense against dangerous combinations, these computer software programs also pose the risk of failing to detect some clinically significant drug interactions while generating excess clinically insignificant alerts that put clinicians at risk for alert fatigue. The more complete the database, the more nonspecific the warnings may become, often promoting a therapeutic paralysis that is functionally equivalent to a total ignorance of drug-drug interactions. Reports of physician override alert rates as high as 90% underscore the need to develop a system to prioritize alerts in a manner that reduces the risk of missing a serious drug interaction.18 Pharmacists can triage the “low clinical value” alerts generated by technology rules and filters that may appear to have heightened importance and can determine whether such alerts are relevant. Some examples of these “nuisance alerts” related to flawed rules include interactions with oral and topical medications, formulation-specific alerts, and time-dependent administration.
Conversely, pharmacists who are less experienced with psychiatric drug interactions may dismiss interactions for discontinued agents, when in fact these interactions may be serious and relevant. Long-acting antidepressants, such as monoamine oxidase inhibitors (MAOIs), and depot-injected antipsychotics can impact potential drug interactions for weeks after they are discontinued.18 Claudia Lee, MD, BS(Pharm), medical physician and registered pharmacist at the Buffalo Psychiatric Center, is concerned about this alert fatigue and recommends that “all physicians should have focused refreshers for medications they use that can have serious consequences as a result of drug interactions that may be dismissed or missed entirely” (Interviewed October 11, 2012).
Individuals with psychiatric disorders are at a high risk for drug interactions and the ADRs associated with these problematic combinations. These individuals often require medication to control their psychiatric condition and frequently need other medications to treat the side effects of the psychiatric drugs. Such side effects include, but are not limited to, movement disorders, metabolic syndrome, thyroid dysfunction, and diminished renal and hepatic function. Drug interactions become more likely and common for these patients as a result of their multiple medication regimens. Further, these patients are at a higher risk of serious adverse medication events and are subject to a significantly premature death from all causes compared to the general population.19 Further complicating the prediction of psychiatric drug interactions is the issue of unique metabolic pathways, which vary with an individual’s genetic polymorphisms, gender, and age. Environmental issues such as smoking, diet, and exposure to toxins and chemicals also cloud the potential predictive capabilities of even the most sophisticated drug-interaction program.
Prescribers are often challenged by the necessity of ordering a medication regimen that poses a known risk of drug-drug interactions in order to achieve a clinically desirable goal. However, whenever possible, it is advisable to use multiple drug therapy only when clearly indicated and with the following cautions in mind. Since most drug interactions are primarily metabolic and are predictable based on knowledge of the isoenzymes involved, it is critical that the team involved in the medication management of hospitalized patients be aware of the most important psychiatric drug interactions, the mechanism responsible for the interaction, and the corrective action required to prevent a negative clinical outcome.20 An example is provided in TABLE 2.11

Conclusion
Chronic coadministration of psychiatric drugs with known interaction potential can be safely managed with dose changes, therapeutic interchanges with similar products, and/or administration time adjustments. Newly initiated combinations are the highest risk for adverse effects from drug interactions; thus, first-dose monitoring is essential, along with the patient’s own personal report of the perceived benefit and side effects of therapy. When considering drug-drug interactions with psychiatric agents, practitioners and prescribers should consider the following survival tips3:
- Become an expert in the drugs used most often in one’s own practice
- Pay special attention to those drugs with narrow therapeutic indices and potential lethal doses
- Consult resources frequently
- Try to select agents that have a low degree of drug-interaction potential.
REFERENCES
1. Thomas M, Boggs AA, DiPaula B, Shadhida S. Adverse drug reactions in hospitalized psychiatric patients. Ann Pharmacother. 2010;44:819-825.
2. McDonnell P, Jacobs M. Hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother. 2002;36:1331-1336.
3. Sandson NB, Armstrong SC, Cozza KL. Med-psych drug-drug
interactions update: an overview of psychotropic drug-drug
interactions. Psychosomatics. 2005;46:464-494.
4. Spina E, Scordo MG, Arrigo C. Metabolic drug interactions with new psychotropic agents. Fundam Clin Pharmacol. 2003;17:517-538.
5. Linnet K, Ejsing TB. A review on the impact of
P-glycoprotein on the penetration of drugs into the brain. Focus on
psychotropic drugs. Eur Neuropsychopharmacol. 2008;18:157-169.
6. Shargel L, Wu-Pong S, Yu A. Applied Biopharmaceutics and Pharmacokinetics. 5th ed. New York, NY: McGraw-Hill; 2005:412-416.
7. French JA, Gidal BE. Antiepileptic drug interactions. Epilepsia. 2000;41(suppl 8):S30-S36.
8. Zevin S, Benowitz N. Drug interactions with tobacco smoking. Clin Pharmacokinet. 1999;36:425-438.
9. Cole M, Trigoboff E, Demler TL, Opler LA. Impact of
smoking cessation on psychiatric inpatients treated with clozapine or
olanzapine. J Psychiatr Pract. 2010;16:75-81.
10. Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64:1912-1921.
11. Clinical Pharmacology [database online]. www.clinicalpharmacology-ip.com. Accessed July 12, 2012.
12. Brown CS, Markowitz JS, Moore TR, Parker NG. Atypical
antipsychotics: Part II: adverse effects, drug interactions, and costs. Ann Pharmacother. 1999;33:210-217.
13. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112-1120.
14. DePonti F, Poluzzi E, Montanar N. QT-interval
prolongation by non-cardiac drugs: lessons to be learned from recent
experience. Eur J Clin Pharmacol. 2003;56:1-18.
15. Moss AJ. The QT interval and torsades de pointes. Drug Saf. 1999;21(suppl 1):5-10.
16. Al-Khatib SM, LaPointe NM, Kramer JM, Califf RM. What clinicians should know about the QT interval. JAMA. 2003;289:2120-2127.
17. Oyesanmi O, Kunkel EJ, Monti DA, Field HL. Hematologic side effects of psychotropics. Psychosomatics. 1999;40:414-421.
18. Allen J, Armeni T. Drug interaction overload: problems and solutions for drug interaction alerts. Pharm Lett. 2012; Course no. 216.
19. Laursen TM, Olsen TM, Nordentoft M, Mortensen PB.
Increased mortality among patients admitted with major psychiatric
disorders: a register-based study comparing mortality in unipolar
disorder, bipolar affective disorder, schizoaffective disorder, and
schizophrenia. J Clin Psychiatry. 2007;68:899-907.
20. Patsalos PN, Perucca E. Clinically important drug
interactions in epilepsy: general features and interactions between
antiepileptic drugs. Lancet Neurol. 2003;2:347-356.
To comment on this article, contact rdavidson@uspharmacist.com.





