US Pharm. 2007;32(12):26-34.
Alzheimer's disease (AD) is
the most common form of dementia in the United States, with more than 5
million patients living with the disease in 2007.1 Many pharmacists
work with AD patients on a regular basis and are familiar with its
pathophysiology, clinical presentation, and pharmacotherapy. Furthermore,
excellent articles reviewing the management of AD are available elsewhere.
2-4 With the increasing longevity in the U.S. population, the number of
patients living with AD or with other, less commonly encountered dementias
will rise. Therefore, community pharmacists should also become familiar with
some of the other types of dementias they are likely to encounter in clinical
practice. This article attempts to summarize the major therapeutically
encountered points of four other major causes of dementia: vascular dementia,
dementia with Lewy bodies, Parkinson's disease with dementia, and
frontotemporal dementia.
Vascular Dementia
Vascular dementia
(VaD), formerly referred to as multi-infarct dementia, is the second most
commonly encountered dementia after AD.5 One case series of over
600 demented patients ages 75 years or older found that 47% of patients had AD
and 23% had VaD.6 Worldwide, approximately 15% to 20% of all
dementias are thought to be due to vascular causes, a figure that is expected
to increase as cerebrovascular disease rates rise.7
VaD frequently coexists with
AD in elderly patients, and this complicates the discussion of the disease. In
fact, it has been suggested that "pure" AD or "pure" VaD simply do not exist
late in life, though this is not necessarily true in younger patients.8
The two diseases share common risk factors, including hypertension, diabetes
mellitus, and cigarette smoking.9 Thus, while it has traditionally
been thought that AD was neurodegenerative in origin and is distinct from the
ischemic pathophysiology of VaD, it is quite possible that the two diseases
exist on a spectrum and are more similar pathophysiologically than is often
believed.
Despite the overlap between AD
and VaD and the fact that a definitive diagnosis can only be made at autopsy,
specific criteria for diagnosing probable VaD do exist.10 To
summarize, a diagnosis of probable VaD requires each of the following: a
decline in cognitive function, including memory, from the patient's previous
baseline; the presence of cerebrovascular disease by neurologic findings
and/or imaging studies; and a temporal relationship between the previous two
findings such as the worsening of cognitive symptoms within three months of a
cerebrovascular event. Though commonly used, these criteria have been
criticized as underdiagnosing VaD, and other diagnostic tools have been
proposed.11
VaD is caused by ischemic
changes to the brain and can be either sudden in onset due to an acute event
or more chronic and caused by ongoing ischemia. Among patients over the age of
65 who suffer an acute cerebrovascular event, approximately 25% will
experience VaD, a reason why poststroke disability is so common.8,12
This also explains the classic stepwise pattern in the loss of cognitive
function in VaD, as patients are relatively stable between ischemic events and
then suffer a sudden, significant loss with recurrent strokes. While
historically only larger, discreet ischemic events were believed to predispose
a patient to VaD, ongoing "silent" ischemia is felt to be an underappreciated
cause of this disorder.13
The clinical presentation of
VaD is comparable to that of AD, though some subtle differences may exist.
Memory disturbances are probably more of an issue with AD than with VaD,
though in either condition they are often what causes patients to seek medical
attention. VaD is more likely to cause gait alterations and personality
changes than AD but is less likely to cause language difficulties.8
Because the pathophysiology of
both VaD and AD involve the loss of cholinergic neurons, the mainstay of
treatment is drugs affecting cholinergic neurotransmission. Several clinical
trials have been performed and published, though most are small, open label,
or of short duration, or suffer from other significant methodological flaws.
The most well-known study was performed by Erkinjutti, et al.,14
who compared galantamine to placebo in nearly 600 patients with VaD or the
combination of AD and cerebrovascular disease. Patients with diagnoses of VaD
accounted for less than half of the study participants, reflecting the
infrequency of "pure" VaD. Those who received galantamine in the six-month
trial experienced less progression of their disease than those who received
placebo, and the degree of benefit was comparable to what has previously been
observed in trials of cholinesterase inhibitors for AD. However, a subgroup
analysis revealed that patients with AD and cerebrovascular disease benefited
somewhat more from treatment than did those with probable VaD. As expected,
gastrointestinal complaints were the most common adverse effects observed in
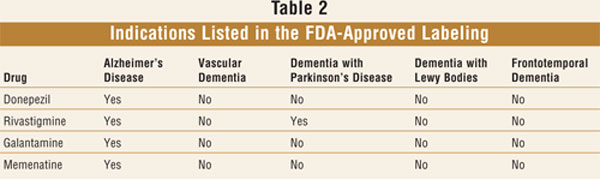
the trial. Clinical trial results with donepezil,15 rivastigmine,
16 and memantine17 have also been largely positive.
Preventing or minimizing
further cerebrovascular ischemia is probably the most important treatment
outcome for patients with VaD. A small study suggested that aspirin therapy
may slow down cognitive decline.18 Guidelines for prevention of
stroke in those with a previous stroke are available (TABLE 1).19

Dementia with Lewy Bodies
and Parkinson's
Disease with Dementia
Dementia with Lewy
bodies (DLB) and Parkinson's disease (PD) with dementia are slightly less
prevalent than VaD6 and differ significantly from AD and VaD in
terms of pathophysiology. The abnormal neuroanatomical structures known as
Lewy bodies were first described in patients with Parkinson's disease by
the German-American physiologist Frederick Lewy in the early 1900s, but an
association between them and dementia was not noted until the 1960s.20
Lewy bodies are distinct from the neuritic plaques and neurofibrillary
tangles characteristic of AD, though older patients who have been diagnosed
with DLB or PD with dementia may have both neuritic plaques and Lewy bodies
present. Neurofibrillary tangles are relatively unusual in DLB and PD with
dementia.21 Though Lewy bodies are present in both DLB and PD with
or without dementia, in DLB they are present in the subcortical and
frontotemporal areas of the brain, while in PD they are located primarily in
the substantia nigra region.21
Some controversy still exists
as to whether DLB and PD with dementia are two separate diseases or just
different manifestations of the same underlying disease process. Most experts
will label patients whose dementia symptoms predate their motor symptoms or
whose motor symptoms were present for less than a year before the onset of
dementia as DLB. Those individuals who have PD for at least a year before
dementia symptoms start are typically labeled as having PD with dementia.
Since the management of the two conditions is, with few exceptions, very
similar, this is largely a matter of semantics.22 The idea that DLB
may be a distinct disease is relatively new and was not put forward until the
1980s.23
Criteria for the diagnosis of
DLB are available.24 The central feature of the condition is the
presence of a progressive loss of cognitive function significant enough to
interfere with normal work or life activities. The core features are 1)
fluctuating cognition with significant variation in alertness; 2) recurrent
visual hallucinations; and 3) symptoms of parkinsonism. Two of these three are
necessary for a probable diagnosis of DLB, and one of the three for a possible
diagnosis. Other suggestive features, supportive features, and features making
a diagnosis of DLB less likely are also listed in the diagnostic criteria, but
it is the core features that truly help display how DLB differs from other
dementias, so these will be discussed in more detail.
The fluctuations in cognitive
function can be significant in DLB. Day-to-day or even hour-to-hour variation
is typical and one of the major ways DLB can be differentiated from AD. AD
patients may have good days and bad days in terms of alertness and cognitive
function, but the variation is typically much less pronounced. Hallucinations
are also prominent in DLB. Typically, patients will have highly detailed
visual hallucinations that, somewhat surprisingly, they will report as not
particularly bothersome. Stereotypically, the hallucinations are of children,
though this does not have to be the case. Finally, the parkinsonian features
including tremor, rigidity, and bradykinesia, are a common finding in patients
with DLB, though the tremor in particular tends to be less pronounced than in
PD.
Though not often useful for
diagnosing DLB, one of the suggestive features of the disease is an
exaggerated sensitivity to neuroleptic agents. As many as half of patients
with DLB and 40% of those with PD with dementia experience severe adverse
reactions to these drugs and other agents that antagonize dopaminergic
receptors.24,25 Many clinicians feel that newer, atypical
antipsychotic agents pose a lower, though not negligible, risk. Because
hallucinations and, to a lesser extent, agitation are common manifestations of
DLB and PD with demenia, this potential sensitivity to neuroleptic agents is
something all pharmacists should be aware of. Given the benign nature of the
visual hallucinations and also that agitation can often be treated with
nonneuroleptic agents such as trazodone, minimizing the use of dopamine
antagonists in patients with DLB or PD with dementia is critical. Patients and
their caregivers should also be informed of the risks associated with
neuroleptics, as many prescribers are unfamiliar with this particular
complication of DLB. Unfortunately, early in the course of disease DLB can be
difficult to differentiate from AD, and it is common that one of the early
clues that a patient may have DLB rather than the more common AD is a severe
adverse reaction to a prescribed neuroleptic.
Few data from clinical trials
exist to help guide treatment of the cognitive dysfunction. One of the more
widely discussed trials was performed by McKeith and colleagues, who
randomized 120 patients with DLB to the cholinesterase inhibitor galantamine
or to placebo.26 At the end of the 20-week trial period, those
randomized to galantamine experienced improvements in hallucinations,
delusions, apathy, and anxiety. Adverse effects, though more common in the
active treatment group, were generally mild and self-limited. Some evidence
suggests that patients with DLB may respond better to cholinesterase
inhibitors than do AD patients, so a trial of one of these agents is warranted
in most individuals.27 Additional trials have shown benefit of a
cholinesterase inhibitor in individuals with PD with dementia, and
rivastigmine's FDA-approved labeling includes the indication of PD with
dementia.28,29 No treatment is currently FDA approved for DLB (
TABLE 2).
Parkinsonian symptoms in
patients with DLB are treated conventionally with a few caveats. Patients may
be at increased risk of adverse effects from anti-parkinsonian drugs, and a
small study suggested that the response to levodopa may be suboptimal.30
Slow initiation and close monitoring are critical. As many individuals with
DLB are already suffering from hallucinations, this particular adverse effect
of antiparkinsonian drugs may be especially troublesome because neuroleptic
therapy, as discussed earlier, is often not an option due to the sensitivity
to these agents.
Frontotemporal Dementia
Frontotemporal
dementia (FTD) is an umbrella term that incorporates more specific disorders
including Pick's disease, primary progressive aphasia (PPA), and others. It is
believed to be responsible for approximately 5% of all dementia cases, though
this figure is much higher in younger (<65 years) patients.31
Unlike AD and other more common forms of dementia, the mean age of onset is
probably the 50s rather than the 60s or beyond as is typical for AD, VaD, and
DLB.32,33 FTD has a stronger genetic component that the other forms
of dementia discussed above, with around 40% of patients having a family
member affected.34 Pathophysiologically, FTD is characterized by
atrophy of the frontal and temporal lobes of the brain noted on magnetic
resonance imaging or other imaging technique, hence the "frontotemporal"
nomenclature.
Particularly when the frontal
lobe itself is primarily involved, FTD is characterized by what is typically
referred to as executive dysfunction. Patients with FTD in whom
executive dysfunction is the major problem are often labeled as having Pick's
disease. Broadly speaking, executive function is the process of responding to
stimuli in a goal-directed manner. The mnemonic SOS-MOMMI is sometimes used to
describe executive dysfunction (TABLE 3).35 Stereotypically,
patients with this form of FTD may display inappropriate behavior (especially
sexual), apathy, loss of empathy, or lack of interest in grooming or
appearance. Although memory loss may not be present early in the course of the
disease, executive dysfunction correlates strongly with poor functional
outcomes in patients with mild cognitive impairment and may result in myriad
problems by itself.36

As expected based on its name,
loss of language function is the defining characteristic in patients with the
PPA form of FTD. Memory issues may also present later in the course of PPA,
however.
Very few clinical studies have
been performed in patients with frontotemporal dementia. Treatment is
generally symptom focused. Agents affecting serotonin, particularly the
selective serotonin reuptake inhibitors and trazodone, are often prescribed if
depression, anxiety, insomnia, or apathy is present.37,38
Compulsive behavior may also be positively impacted by serotonin reuptake
inhibitor use. Aggression or agitation is often treated with antipsychotics,
but caution should be used, as extrapyramidal symptoms may develop as the
disease progresses.39 Cholinesterase inhibitors and memantine have
not been well studied in this population, but clinical experience has shown
that most patients do not substantially benefit. Because of the overall
disappointing results with drug therapy, nonpharmacologic interventions
against FTD, such as patient education, redirection, and caregiver support,
continue to be the mainstays of treatment.

Summary and Conclusion
Demographic changes
and increasing life expectancy due to continued improvements in medical care
all but ensure that pharmacists will be assisting in the care of more demented
patients in the future. While AD, VaD, DLB, PD with dementia, and FTD share
many common characteristics and the treatments often overlap, there are subtle
differences and special points with each condition of which all pharmacists
should be aware (TABLE 4). Ensuring that patients are receiving
appropriate cerebrovascular risk reduction therapies in VaD and avoiding the
use of neuroleptic agents in those with DLB or PD with dementia are two areas
in particular where pharmacists can have a major impact on patient care. Being
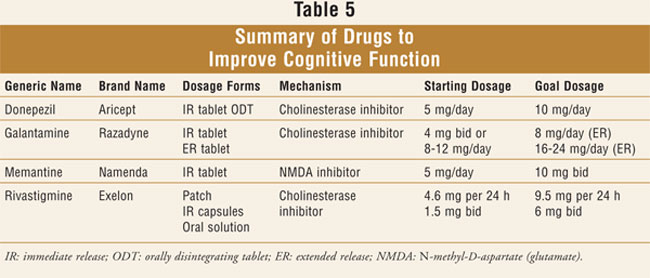
familiar with the drugs used to improve cognition will also be helpful (
TABLE 5). Hopefully, the next several years will produce much more
published research and lead to better decisions about treatment of dementias.
References
1. Alzheimer's
Association. Alzheimer News. 3/20/207.
http://www.alz.org/news_and_events_rates_rise.asp Accessed September 23, 2007.
2. Cummings JL.
Alzheimer's disease. N Engl J Med. 2004;351:56-67.
3. Blennow K, de Leon
MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387-403.
4. Farlow MR, Cummings
JL. Effective pharmacologic management of Alzheimer's disease. Am J Med
. 2007;120:388-397.
5. Roman GC. Stroke,
cognitive decline and vascular dementia: the silent epidemic of the 21st
century. Neuroepidemiology 2003;22:161-164.
6. Rahkonen T,
Eloniemi-Sulkava U, Rissanen S, et al. Dementia with Lewy bodies according to
the consensus criteria in a general population aged 75 years or older. J
Neurol Neurosurg Psychiatry. 2003;74:720-724.
7. O'Brien J, Ames D,
Gustafson L, et al, eds. Cerebrovascular Disease, Cognitive Impairment and
Dementia. 2nd ed. London, England: Martin Dunitz; 2004:95-100.
8. Roman GC. Facts,
myths, and controversies in vascular dementia. J Neurological Sci.
2004;226:49-52.
9. Iadecola C, Gorelick
PB. Converging pathogenic mechanisms in vascular and neurodegenerative
dementia. Stroke. 2003;34:335-337.
10. Roman GC, Tatemichi
TK, Erkinjutti T, et al. Vascular dementia: diagnostic criteria for research
studies. Report of the NINDS-AIREN International Workshop. Neurology.
1993;43:250-260.
11. Lopez OL, Kuller
LH, Becker JT, et al. Classification of vascular dementia in the
Cardiovascular Health Study Cognition Study. Neurology.
2005;64:1539-1547.
12. Barba R,
Martinez-Espinosa S, Rodrõguez-Garcõa E, et al. Poststroke dementia: clinical
features and risk factors. Stroke. 2000;31:1494-1501.
13. Vermeer SE, Den
Heijer T, Koudstaal PJ, et al. Incidence and risk factors of silent brain
infarcts in the population-based Rotterdam Scan Study. Stroke.
2003;34:392-396.
14. Erkinjutti T, Kurz
A, Gauthier S, et al. Efficacy of galantamine in probable vascular dementia
and Alzheimer's disease combined with cerebrovascular disease: a randomized
trial. Lancet. 2002;359:1283-1290.
15. Roman GC, Wilkinson
DG, Doody RS, et al. Donepezil in vascular dementia: combined analysis of two
large-scale clinical trials. Dement Geriatr Cogn Disord.
2005;20:338-344.
16. Moretti R, Torre P,
Antonello RM, et al. Rivastigmine in subcortical vascular dementia: a
randomized, controlled, open 12-month study in 208 patients. Am J
Alzheimers Dis Other Demen. 2003;18:265-272.
17. Mobius HJ, Stoffler
A. Memantine in vascular dementia. Int Psychogeriatr. 2003;15(suppl
1):207-213.
18. Meyer JS, Rogers
RL, McClintic K, et al. Randomized clinical trial of daily aspirin therapy in
multiinfarct dementia. A pilot study. J Am Geriatr Soc.
1989;37:549-555.
19. Sacco RL, Adams R,
Albers G, et al. Guidelines for prevention of stroke in patients with ischemic
stroke or transient ischemic attack. Stroke. 2006;37:577-617.
20. Geldmacher DS.
Dementia with Lewy bodies: diagnosis and clinical approach. Cleve Clin J Med
. 2004:71:789-800.
21. Neef D, Walling AD.
Dementia with Lewy bodies: an emerging disease. Am Fam Physician.
2006;73:1223-1229.
22. McKeith IG, Dickson
DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies:
third report of the DLB Consortium. Neurology. 2005;65:1863-1872.
23. Kosaka K, Yoshimura
M, Ikeda K, et al. Diffuse type of Lewy body disease: progressive dementia
with abundant cortical Lewy bodies and senile changes of varying degree--a new
disease? Clin Neuropathol. 1984;3:185-192.
24. McKeith I,
Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile
dementia of Lewy body type. BMJ. 1992;305:673-678.
25. Aarsland D, Perry
R, Larsen JP, et al. Neuroleptic sensitivity in Parkinson's disease and
parkinsonian dementias. J Clin Psychiatry. 2005;66:633-637.
26. McKeith I, Del Ser
T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a
randomised, double blind, placebo-controlled international study. Lancet
. 2000;356:2031-2036.
27. Samuel W, Caliguri
M, Galasko D, et al. Better cognitive and psychopathologic response to
donepezil in patients prospectively diagnosed as dementia with Lewy bodies: a
preliminary study. Int J Geriatr Psychiatry. 2000;15:794-802.
28. Emre M, Aarsland D,
Albanese A, et al. Rivastigmine for dementia associated with Parkinson's
disease. N Engl J Med. 2004;351:2509-2518.
29. Ravina B, Putt M,
Siderowf A, et al. Donepezil for dementia in Parkinson's disease: a
randomised, double blind, placebo controlled, crossover study. J Neurol
Neurosurg Psychiatry. 2005;76:934-939.
30. Molloy S, McKeith
IG, O'Brien JT, et al. The role of levodopa in the management of dementia with
Lewy bodies. J Neurol Neurosurg Psychiatry. 2005;76:1200-1203.
31. Ratnavalli E,
Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia.
Neurology. 2002;58:1615-1621.
32. Kertesz A, Munoz D,
eds. Pick's Disease and Pick Complex. New York, NY: Wiley-Liss;
1998:23ñ33.
33. Westbury C, Bub D.
Primary progressive aphasia: a review of 112 cases. Brain Lang.
1997;60:381-406.
34. Stevens M, van
Duijn CM, Kamphorst W, et al. Familial aggregation in frontotemporal dementia.
Neurology. 1998;50:1541-1545.
35. Hill RD, Backman L,
Neely AS, eds. Cognitive Rehabilitation in Old Age. New York, NY:
Oxford University Press; 2000:159-173.
36. Royall DR, Chiodo
LK, Polk MJ. Correlates of disability among elderly retirees with
"sub-clinical" cognitive impairment. J Gerontol Med Sci.
2000;5A:M541ñM546.
37. Moretti R, Torre P,
Antonello RM, et al. Frontotemporal dementia: paroxetine as a possible
treatment of behavior symptoms. A randomized, controlled, open 14-month study.
Eur Neurol. 2003;49:13-19.
38. Lebert F, Stekke W,
Hasenbroekx C, Pasquier F. Trazodone in fronto-temporal dementia. Res Pract
Alzheimer's Dis.2006;11:356-360.
39. Mariani C, Defendi
S, Mailand E, Pomati S. Frontotemporal dementia. Neurol Sci. 2006.
To comment on this article,
contact editor@uspharmacist.com.






