US Pharm. 2007;32(7)(Oncology suppl):11-24.
ABSTRACT: Therapeutic cancer vaccines
differ from traditional vaccines in that they are only given to patients who
already have cancer, with the goal of decreasing the spread of the disease and
prolonging survival. Such vaccines are designed to stimulate the immune system
to selectively kill tumor cells. While therapeutic prostate cancer vaccines
have not yet received FDA approval, many have shown promising activity in
clinical trials. This article reviews the etiology, pathophysiology,
diagnosis, and treatment of prostate cancer and explores the potential roles
of three therapeutic prostate cancer vaccines: PROSTVAC-VF, Provenge, and GVAX.
Over the last decade, significant progress
has been made in the diagnosis and treatment of prostate cancer.1
Today, prostate cancer can be detected earlier with the use of routine
prostate-specific antigen (PSA) screenings.1,2 As a result,
curative modalities offer better success rates. Treatment options for advanced
disease are also better; hormonal therapy is commonly initiated earlier in the
course of the disease, and more effective chemotherapeutic regimens that
prolong survival are now being used.2,3 According to the American
Cancer Society (ACS), these factors have contributed to a decrease in the
mortality rate of prostate cancer of 3.5% annually in recent years.4
Despite these positive trends, prostate cancer still ranks as the second
leading cause of cancer-related deaths in American men.1,2,4
Recent advances in
understanding the pathophysiology of prostate cancer have led to the discovery
of numerous potential therapeutic prostate cancer vaccines.5,6
While these products have not yet been approved for use in the United States,
many have demonstrated promising activity in phase II and III clinical trials.
2,5,7
Prostate Anatomy and
Physiology
The prostate, a
walnut-sized gland located in the pelvis, is surrounded by the rectum,
bladder, urinary sphincter, and penile innervation.8-12 The
prostate is composed of three different cell types: stromal cells, glandular
cells, and smooth muscle cells. The glandular cells within the prostate
produce a milky fluid, and during sexual intercourse, the smooth muscles
contract to squeeze this fluid into the urethra. At this point, the fluid
mixes with sperm and other fluids to make semen. The prostate gland also
contains an enzyme called 5 alpha-reductase, which is responsible for
converting testosterone to dihydrotestosterone.4,8,9
Prostate Cancer
Prostate cancer is
the most common cancer in American males and the fourth most common cancer
worldwide.1,4,10,11 About 1 in 6 men will be diagnosed with
prostate cancer during his lifetime, but only 1 in 34 will die from it. The
ACS estimates that in 2007, approximately 210,000 new cases of prostate cancer
will be diagnosed, and 27,000 men will die from the disease. More than 99% of
prostate cancers develop in glandular cells and are termed adenocarcinomas
.4 Generally speaking, the survival rate for prostate cancer is
excellent when the cancer is detected early.9
Causes and Risk Factors:
While the exact cause of prostate cancer is unknown, data suggest that several
factors may contribute to the development of the disease. Prostate cancer is
more common in older men, with 75% of new prostate cancers diagnosed after age
65. African-Americans are 1.7 times more likely to develop prostate cancer
than white Americans.10 In addition, prostate cancer occurs less
often in Asian men than in white men, and Hispanic men develop prostate cancer
at rates similar to those of white men; the exact reasons for these racial
differences are not clear.4 Family history also appears to have a
role. Men who have a first-degree relative (i.e., a father, brother, or son)
with prostate cancer are twice as likely to develop the disease, compared with
men who have no family history of prostate cancer. Other factors that may
increase the risk of prostate cancer include exposure to industrial chemicals,
high-fat diets, and high testosterone levels.10
Prevention:
Limited data suggest that certain lifestyle changes and medications can
prevent some cases of prostate cancer. Prostate cancer rates are lower in
populations that have low-fat, plant-based diets. Furthermore, higher fat
intake is associated with an increased risk of prostate cancer.12
Products such as tomatoes, pink grapefruit, and watermelon, which are rich in
lycopenes--antioxidants that help prevent damage to DNA--may also lower prostate
cancer risk slightly.4 Likewise, data suggest that certain vitamin
and mineral supplements (e.g., selenium and vitamin E) may decrease prostate
cancer risks. Perhaps the most convincing piece of evidence on this topic
comes from the Prostate Cancer Prevention Trial. In this study, men who took
finasteride (Proscar) were about 25% less likely to develop prostate cancer
than those who took placebo.4,8
Symptoms:
Prostate cancer usually does not cause symptoms in the early stages of the
disease.9 However, as the malignancy spreads, it may constrict the
urethra and cause urinary problems similar to those seen in benign prostatic
hyperplasia.4,9,12 Locally advanced disease can invade adjacent
tissues, including the seminal vesicles and bladder. Urinary
dysfunction--decreased urine stream, inability to urinate, blood in the urine,
interruption in the urine stream, frequent urination (especially at night),
and pain and burning during urination--and new-onset impotence are symptoms of
locally advanced prostate cancer.12 The primary symptoms associated
with late-stage prostate cancer usually include significant pain in one or
more bones. This chronic pain occurs most often in the spine and sometimes
flares in the pelvis, lower back, hips, or bones of the upper legs. In many
cases, the chronic pain may also be accompanied by significant weight loss.
9
Detection: Since
the early 1900s, digital rectal exams have been the primary tool for detection
of prostate cancer.12 Although about 90% of all prostate cancers
arise in the outer part of the prostate near the rectum, only a small portion
can be detected by digital rectal exams. The exam is quick and painless, but
many men find it to be extremely embarrassing.9 Although digital
rectal exams carry a specificity for prostate disease of greater than 85%,
they should not be used alone as a screening tool.12
Screening for prostate cancer
was enhanced in the late 1980s with the introduction of an immunoassay for PSA.
9,12 Prostate cancer cells appear to release PSA into the bloodstream in
elevated quantities.9 Levels of PSA greater than 4 ng/mL are more
likely associated with prostate cancer.4 Since the introduction of
PSA screening in the 1980s, there has been a dramatic increase in the
incidence of prostate cancer in the U.S. Before the availability of PSA
testing, only a minority (25%) of cancers detected were confined to the
prostate gland. Because of the widespread use of PSA screening, the majority
(75%) of prostate cancers now discovered are confined to the prostate gland.
12 However, the PSA test is far from foolproof. Nonmalignant causes of
an elevated PSA level include presence of benign prostatic hyperplasia or
prostatitis, increasing age, ejaculation within two days of the blood test,
recent digital rectal exams or prostate biopsies, and use of finasteride. The
PSA test is not accurate enough to completely rule out or confirm the presence
of cancer. Relying too heavily on the test may lead to unnecessary biopsies,
while not relying on it carries the risk of the cancer being undetected.9
Recommendations from the ACS with regard to PSA screenings and digital rectal
exams are discussed in Table 1.9,12

Diagnosis: A
prostate biopsy is necessary to confirm a diagnosis of prostate cancer and to
grade the tumor specimen. Ten to 12 samples are usually taken during a
prostate needle biopsy using a transrectal approach with ultrasound guidance.
4,11
Prostate cancers are most
commonly graded according to the Gleason system. This system assigns a Gleason
grade, using numbers from 1 to 5, based on how much the cells in the cancerous
tissue resemble normal prostate tissues. Grade 1 tumors resemble normal
prostate cells, whereas grade 5 tumors have cells that seem to be poorly
organized. Grade 2, 3, and 4 tumors have features between these extremes.
Because prostate cancers often have areas with different grades, the two most
predominant grades are added together to yield a Gleason score between 2 and
10. The higher the Gleason score, the more likely the cancer will grow and
spread quickly.4
The tumor-node-metastasis
(TNM) classification system (Table 2),13 the preferred
staging system developed by the American Joint Committee on Cancer, is updated
every five years to include new pathologic findings. Staging in the TNM system
is based on tumor size (T), nodal status (N), and presence or absence of
metastasis (M). Similar TNM staging systems have been developed for most other
cancers.12 The prognosis for patients with prostate cancer depends
on many factors, including the TNM grade, Gleason score, tumor volume, PSA,
and patient age.11

Conventional Treatments of
Prostate Cancer
Conventional
treatment options for prostate cancer require assessing patients' individual
needs with respect to life expectancy, comorbidities, likelihood of a cure,
and personal choice based on the potential adverse effects of each treatment.
The initial treatment for prostate cancer depends on several factors,
including the TNMgrade, the Gleason score, PSA, presence of distant disease,
and presence of symptoms.11,12 Treatments for men with localized
prostate cancer include watchful waiting (also known as expectant management
), surgical prostate removal, radiotherapy, and other ablation. Androgen
deprivation may be used for men who wish to receive some therapy but are not
candidates for or do not wish to receive surgery or radiation. Androgen
deprivation therapy is considered the mainstay of treatment for men with
advanced prostate cancer; improvement in symptoms and disease regression in
over 80% of patients has been noted. For hormone-refractory prostate cancer,
other treatments, such as chemotherapy, are warranted.14
Watchful Waiting:
Expectant management is often used for slow-growing tumors that are
asymptomatic, especially in older men. Watchful waiting incorporates routine
follow-up monitoring with intervention if the disease progresses or symptoms
develop. Patients usually receive digital rectal exams and PSA screenings
every six months during the observation. This treatment option allows patients
to avoid unnecessary surgery or treatments that may affect quality of life.
12
Surgery and
Radiotherapy: Surgery or radiotherapy may be recommended for low-risk,
early stage prostate cancer (T1 or T2) who have a life expectancy of more than
10 years and no significant comorbidities.12,14 Radical
prostatectomy and radiotherapy are considered therapeutic equivalents for
treatment of low-risk prostate cancer. Radical prostatectomy consists of
removing the prostate gland and seminal vesicles.12 This surgery
can be done as the standard "open" procedure or by the newer laparoscopic or
robotic techniques. Advantages to laparoscopic or robotic radical
prostatectomy include shorter hospital stays, smaller incisions, decreased
postoperative pain, and a potential decreased risk of both incontinence and
erectile dysfunction.15
External beam radiotherapy is
used with either conventional technology or three-dimensional conformal
radiotherapy or intensity-modulated radiotherapy. The newer techniques allow
higher doses to reach target tissue with less toxicity.12,14,16
Brachytherapy is another option for treatment of localized prostate cancer. It
involves the insertion of permanent or temporary radioactive seeds directly
into the gland to deliver focused radiation and minimize toxicity to normal
tissues.12,14 Ultrasound and computed tomography scans can be used
to provide more accurate positioning of the seeds.16 Brachytherapy
can also be combined with external beam radiation for treatment of more
advanced disease.14 Patients should avoid contact with pregnant
women and children while the seeds are implanted to avoid unnecessary
radiation exposure. Adverse effects associated with radiotherapy can include
impotence, incontinence, diarrhea, skin irritation and atrophy, fatigue,
urinary frequency, and radiation cystitis. Radiotherapy can also be used after
radical prostatectomy to reduce the risk of disease recurrence.12
Cryosurgery:
Cryosurgery is another therapeutic option for localized prostate cancer. This
technique involves freezing prostate cancer cells using percutaneous probes
filled with liquid nitrogen that is compressed and cooled to -206°C.
Transrectal ultrasound is used to monitor the freezing process and assist with
freezing of the prostate while reducing damage to surrounding normal tissues.
1 Cryosurgery is less established than prostatectomy and long-term
outcomes are less known. Adverse effects of cryosurgery include incontinence,
impotency, and rectal and bladder injury.17 Cryosurgery can also be
used for treatment of patients with more advanced disease, local recurrence,
or salvage therapy after radiation.1,12
High-Intensity Focused
Ultrasound: High-intensity focused ultrasound is an emerging treatment
for localized prostate cancer, as well as a salvage therapy after radiation.
The procedure employs transrectal ultrasound of the prostate that is highly
focused to create an intense heat that kills prostate cancer tissue. Because
the surgeon is able to precisely ablate the gland with pinpoint accuracy, the
adjacent structures are less likely to be affected. The most common side
effects of high-intensity focused ultrasound are obstruction and radiation
necrosis of the urethra. Incontinence is rare, and impotence rates seem to be
low, compared to those seen with other therapies. This procedure is currently
used in Europe, China, Japan, Mexico, Latin America, and the Caribbean and is
undergoing approval by the FDA in the U.S.18
Androgen Deprivation
Therapy: In men with early stage prostate cancer, androgen deprivation
can be used as primary therapy or as an adjunct to other local treatments.
19 Both local and metastatic prostate cancer are under the control of
androgenic hormones.12 Androgen deprivation therapy can be
accomplished by removing the testes, which produce most of the body's
circulating testosterone, or by drug therapy. Effective agents include
luteinizing hormone-releasing hormone (LHRH) agonists, antiandrogens, and
estrogens.12,20
Surgical removal of the
testes, known as bilateral orchiectomy, reduces circulating
testosterone to less than 50 ng/dL. Many patients with prostate cancer are not
surgical candidates due to advanced age, and some may find this treatment
unacceptable. Bilateral orchiectomy is the preferred initial therapy in
patients with spinal cord compression or ureteral obstruction.11,12
LHRH agonists work by
stimulating the anterior pituitary gland to release luteinizing hormone (LH)
and follicle-stimulating hormone. These drugs include leuprolide, goserelin,
histrelin, and triptorelin (Table 3).11,21 High levels of
LHRH agonists inhibit the release of gonadotropins by causing down-regulation
of receptors and a negative feedback loop in the pituitary gland. Levels of
testosterone initially increase and then rapidly decrease to very low levels.
The initial rise in testosterone levels can cause hot flashes, bone pain, and
spinal cord compression during the first week of therapy. This reaction is
known as the flare effect and may be attenuated by using combined
androgen blockage.11,12 The response rate for LHRH agonists is
approximately 80% for advanced prostate cancer. Patients with inadequate
responses may be treated with palliative radiation, additional hormone
therapy, or systemic chemotherapy.12 The LHRH agonists have similar
adverse effects, including erectile dysfunction, hot flashes, loss of libido,
and gynecomastia. Prolonged therapy may cause muscle wasting and osteoporosis,
which can lead to bone fractures. Patients should have baseline and periodic
bone mineral density checks and should receive calcium and vitamin D
supplementation. Bisphosphonates can be used for osteoporosis treatment as
well as for treatment of metastatic bone lesions associated with prostate
cancer.11,12,19,22,23

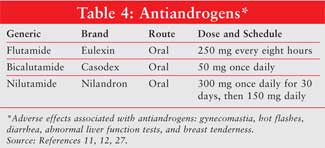
Antiandrogens, including
flutamide, bicalutamide, and nilutamide (Table 4), block androgen
receptors and prevent the body from responding to its own hormones.
11,12,21 Possible adverse effects with the antiandrogens include hot
flashes, gastrointestinal disturbances, abnormal liver function tests, breast
tenderness, and gynecomastia. These agents are associated with less sexual
dysfunction, fewer hot flashes, and less osteoporosis but more gynecomastia
than the LHRH agonists. Antiandrogens can be used as monotherapy but are
usually combined with LHRH agonists.12,19,20
Combined androgen blockade
utilizes LHRH agonists in conjunction with antiandrogen therapy. The
antiandrogens should be initiated one to two weeks prior to the LHRH agonist
to prevent the disease flare caused by the LHRH agonist. The two drugs can be
given together for one to two months or until the prostate cancer progresses.
Combined androgen blockade has been shown to increase progression-free
survival and overall survival in patients with advanced prostate cancer who
are newly diagnosed.12
Estrogen therapy with diethylstilbestrol was once a mainstay of treatment for advanced prostate cancer. However, diethylstilbestrol was removed from the market in 1997 due to increased cardiovascular risk.11 Estrogen therapy is now rarely used for the treatment of prostate cancer.20
Chemotherapy: In
the past, chemotherapy has been considered to be relatively ineffective for
treatment of hormone-refractory prostate cancer or androgen-independent
disease. The standard of care changed in 2004 with the publication of the
TAX-327 trial, which showed that patients with metastatic hormone-refractory
prostate cancer who received docetaxel every three weeks with prednisone had
longer survival, higher response rates, and better pain control than those who
received mitoxantrone and prednisone.20 In general, the type of
chemotherapy that is chosen for a particular patient is based on concomitant
disease states and physician or patient preference. Mitoxantrone should be
avoided in patients with cardiovascular problems, due to its potential to
cause heart failure and arrhythmias. Docetaxel should be avoided in patients
with neurologic problems. Systemic chemotherapy is probably best for patients
with no liver metastases and with mild-to-moderate bone pain.12
Therapeutic Vaccines for
Prostate Cancer
Immunotherapy for
prostate cancer is an active field of investigation using a wide variety of
approaches.22,23 Currently, clinical trials are underway to test
prostate cancer vaccines that are based on genetically modified viruses (e.g.,
PROSTVAC-VF), protein- or peptide-pulsed dendritic cells (e.g, Provenge), or
tumor cells that are modified to secrete proinflammatory cytokines (e.g.,
GVAX). While each of these approaches has unique advantages and disadvantages,
they all endeavor to stimulate the immune system to actively reject prostate
cancer cells.24
PROSTVAC-VF:
PROSTVAC-VF (recombinant vaccinia virus expressing human PSA), a viral vector
vaccine, stimulates the immune system to destroy PSA-expressing cancer cells
by mimicking the natural infection and thereby inducing a potent immune
response.7,25 With this technology, genes that are overexpressed in
a tumor, contain tumor-associated antigens of interest, or express
costimulatory proteins are inserted into viral vectors. These viral vectors
then stimulate antigen-presenting cells. Early clinical data suggest that
viral vaccines have a favorable safety profile and induce a specific T-cell
response.25
PROSTVAC-VF consists of two
genetically engineered vaccines, a partially attenuated version of the virus
used for the smallpox immunization (PROSTVAC-V) and fowlpox virus (PROSTVAC-F)
administered in a sequential regimen.25 Interestingly enough, the
poxviruses are among the most commonly studied vectors for gene delivery.
26 In particular, fowlpox, which is unable to replicate in human cells,
has been shown to be an effective means of boosting cellular immune responses
initiated with vaccinia.25
Further attempts at improving
the PROSTVAC-VF vaccine have included the addition of three costimulatory
molecules (B7-1, ICAM-1, and LFA-3) called TRICOM and
granulocyte-macrophage colony-stimulating factor (GM-CSF).24-26
Data suggest that TRICOM enhances T-cell stimulation, whereas GM-CSF helps
increase tumor-specific immunity and may have some cytotoxic effects.7,24
Numerous phase II and III
studies conducted by the National Cancer Institute have demonstrated the
safety and potential activity of PROSTVAC-VF in patients with different stages
of prostate cancer, including patients with newly diagnosed, localized
disease, patients with biochemical recurrence after hormone therapy without
metastatic disease, and patients with metastatic prostate cancer.22,24
In these studies, injection site reactions and fatigue were the most commonly
reported adverse events.23,24
Researchers are currently
recruiting participants for a phase III trial (known as the PARADIGM
study) that will further examine the safety and potential activity of
PROSTVAC-VF. The PARADIGM study is a randomized, double-blind, controlled
phase III study involving men with prostate cancer who have elevated PSA
levels and no measurable metastatic disease.24,27 The study will
utilize the vaccinia virus, followed by fowlpox schedule in combination with
TRICOM and GM-CSF, administered as a subcutaneous injection on days 1 to 4.
24 The primary efficacy end point will be time to overt metastatic
disease. The FDA recently awarded PROSTVAC-VF with Fast Track designation in
conjunction with the design of the PARADIGM study.27
Provenge:
Sipuleucel-T (Provenge) is also being studied in advanced prostate cancer.
With this therapy, dendritic cells of the immune system are removed from a
patient by leukophoresis. These cells are shipped to a central location where
they are combined with a fusion protein, consisting of prostatic acid
phosphatase and GM-CSF. These cells are matured and activated in vitro and
then sent back for injection into the patient. Sipuleucel-T stimulates the
immune system (specifically T-cell immunity) against prostatic acid
phosphatase, which is present in approximately 95% of prostate cancers.
28-30
Several phase II and III
clinical trials have demonstrated the safety and efficacy of sipuleucel-T. In
a combined phase I/II trial, 31 men with hormone-refractory prostate cancer
received sipuleucel-T. Six patients had significant PSA decreases. No
significant adverse effects were seen.22,31 In a phase II trial
with 21 patients who had metastatic hormone-refractory prostate cancer, one
patient demonstrated a complete response, with a PSA that declined to
undetectable levels. The PSA has remained undetectable for four years. Two
additional patients had transient reductions in PSA levels.31 A
phase III trial examined 127 men with asymptomatic hormone-refractory prostate
cancer who received sipuleucel-T or placebo every two weeks for a total of
three doses. Overall survival was prolonged to a significant degree in
patients who received sipuleucel-T. After three years, 34% of patients who
received sipuleucel-T were alive, compared with 11% of those who received
placebo. The vaccine was generally well tolerated. Side effects more commonly
associated with sipuleucel-T included rigors, pyrexia, tremor, and a "cold"
feeling.31,32 A phase III trial is now underway to evaluate
sipuleucel-T versus placebo in patients with prostatic acid
phosphatase–expressing prostate cancer who have disease-related pain and
disease progression.31 In November 2005, the FDA granted Fast Track
status to sipuleucel-T for the treatment of metastatic hormone-refractory
prostate cancer.5,33
One possible disadvantage to
this vaccine is the great cost and effort involved with its production. Large
amounts of peripheral blood mononuclear cells obtained by leukapheresis must
be cultured for several days in the presence of costly cytokines (e.g.,
GM-CSF, interleukin-4, or tumor necrosis factor-alpha) and then reinfused into
the patient. This labor-intensive approach must be performed for each patient.
22
GVAX: Prostate
GVAX is a type of active immunotherapy. This vaccine uses whole-cell allogenic
prostate cancer cell lines (PC-3 and LnCap), which are genetically modified to
secrete GM-CSF. The tumor cells are irradiated to prevent further cell
division (i.e., tumor growth) before they are injected intradermally.7,34
GM-CSF is an ideal vaccine component due to its ability to effectively
activate dendritic cell antigen presentation. In addition, GM-CSF assists in
the initiation of danger signals that activate the immune system, break the
development of tolerance, and facilitate an antitumor immune response.35
The PC-3 and LnCap cell lines were chosen based on complementary antigenic
features that represent the spectrum of prostate cancer. The PC-3 cell line is
from a prostate cancer bone metastasis and is hormone refractory. This cell
line expresses high levels of several proteases and neuroendocrine peptides,
which are associated with hormone-refractory prostate cancer. The LnCap line
is from a prostate cancer lymph node metastasis expressing antigens such as
PSA and a prostate-specific membrane antigen. The LnCap component is hormone
sensitive.35 One advantage to GVAX is that the vaccine can be used
off-the-shelf in multiple patients.28 In May 2006, the FDA granted
Fast Track status to GVAX for the treatment of advanced prostate cancer.
5,36
Like PROSTVAC-VF and
sipuleucel-T, GVAX has been studied in several phase II and III clinical
trials. The G-9803 trial (phase II) enrolled 34 patients with
hormone-refractory prostate cancer who had asymptomatic metastatic disease.
These patients received 13 doses of GVAX. The median survival for the patients
in the study was 26.2 months--beyond that expected for chemotherapy results or
observation alone.3,35 The most common side effect during this
study was injection site reactions.7 The G-0010 study (phase II)
enrolled 80 patients with asymptomatic metastatic hormone-refractory prostate
cancer.37 Patients received three different doses of GVAX. The
overall survival of the patients in the trial was more than 24.4 months. The
vaccine was well tolerated; common side effects included injection site
reactions, fatigue, malaise, myalgias, and arthralgias.28
Two phase III trials, Vital-1 and Vital-2,
are currently underway. Vital-1, initiated in July 2004, is enrolling
asymptomatic patients who have never received chemotherapy. It compares GVAX
to docetaxel plus prednisone. Vital-2 is enrolling patients with symptomatic
hormone-refractory prostate cancer who have cancer-related pain. This trial
will compare docetaxel plus GVAX with docetaxel plus prednisone.
7,28,34,37,38
Place in Therapy: The vast
majority of patients diagnosed with prostate cancer have localized disease,
which can be treated successfully with surgery or radiation. Despite
treatment, about one third of patients will have advancing disease and require
manipulation of hormones with LHRH agonists and antiandrogens.2,4,12,39
As previously discussed, such therapies are commonly associated with sexual
dysfunction, bone loss, and other embarrassing adverse effects (e.g.,
gynecomastia and hot flashes). In addition, once patients become resistant to
hormonal therapies, cytotoxic chemotherapy agents (which are associated with
nausea and vomiting, myelosuppression, alopecia, and renal toxicities) are
often used.12 In contrast, the prostate cancer vaccines that are
being studied in the treatment of advanced prostate cancer appear to be
extremely efficacious, and few adverse effects have been associated with those
studied in clinical trials. Given their favorable toxicity profile, vaccine
immunotherapy represents a promising new approach for prostate cancer
treatment, either alone or in combination with traditional treatment options.
7,24
Role of the Pharmacist
Pharmacists should
continually remind patients of the importance of annual prostate screenings.
With annual screening, prostate cancer can usually be detected before it
spreads. Patients should be informed that when prostate cancer is detected
early, five-year relative survival rates approach 100%.39
Pharmacists should remind patients that the digital rectal exam takes only
minutes to perform, and the PSA immunoassay is simply a blood test.4
Erectile dysfunction and/or
incontinence are common adverse effects associated with traditional prostate
cancer treatments. When prostate cancer vaccines are combined with traditional
treatment options, pharmacists should communicate with patients about problems
with sexual dysfunction and/or incontinence and discuss both drug and nondrug
treatments.12 Finally, pharmacists should encourage eligible
patients to participate in clinical trials. Patients should be aware that
while these vaccines are still being tested in clinical trials, the
preliminary data is very promising.5,12
References
1. Walsh PC, Retic AB, et al. Campbell's Urology. 8th ed. Philadelphia: Saunders; 2003.
2. Carducci MA, Slain KM. Emerging therapies for prostate cancer CME. The Prostate Cancer Foundation's Report to the Nation on Prostate Cancer 2004. Available at: www.prostatecancerfoundation.org/atf/cf/%7B705B3273-F2EF-4EF6-A653-E15C5D8BB6B1%7D/PCF%20Monograph-final1.pdf. Accessed May 1, 2007.
3. Beer TM. Prostate cancer: risk factors, prognostic indicators, and treatment advances. Presented at: American Society of Clinical Oncology; May 2005; Orlando, FL.
4. All about prostate cancer. American Cancer Society. Available at: www.cancer.org/docroot/CRI/CRI_2x.asp?sitearea=LRN&dt=36. Accessed May 1, 2007.
5. Guthrie EW. Prostate cancer vaccines--Hype or hope. Pharmacy Today. 2007;13:20.
6. Sela M, Hilleman MR. Therapeutic vaccines: realities of today and hopes for tomorrow. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14559.
7. Brand TC, Tolcher AW. Management of high risk metastatic prostate cancer: the case for novel therapies. J Urol. 2006;176:S76-S80.
8. Scher HI. Hyperplastic and malignant diseases of the prostate. In: Kasper DL, Braunwald E, et al., eds. Harrison's Principles of Internal Medicine. 16th ed. New York: McGraw-Hill; 2005:543-552.
9. Prostate cancer patient information. St Louis, MO: MD Consult; 2007. Updated December 15, 2003.
10. Prostate cancer. In: Koda-Kimble MA, Young LY, et al., eds. Applied Therapeutics: The Clinical Use of Drugs . 8th ed. Baltimore: Lippincott Williams and Wilkins; 2005:91-18–91-22.
11. Kolesar JM. Prostate Cancer. In: Dipiro JT, Talbert LT, et al., eds. Pharmacotherapy: A Pathophysiologic Approach. 6th ed. New York: McGraw-Hill; 2005:2421-2435.
12. Tortorice PV. Prostate cancer . In: Pharmacotherapy Self-Assessment Program. 5th ed. American College of Clinical Pharmacy; 2004:123-140.
13. Prostate cancer staging information. National Cancer Institute. Available at: www.cancer.gov/cancertopics/pdq/treatment/prostate/HealthProfessional/page3#Section_18. Accessed May 1, 2007.
14. Small E. Prostate Cancer. In: Goldman L, Ausiello D, eds. CECIL Textbook of Medicine [book on CD-ROM] . 22nd ed. Philadelphia: W.B. Saunders Company; 2004.
15. Intuitive Surgical-A Minimally Invasive Option for the Treatment of Prostate Cancer. Available at: www.intuitivesurgical.com/patientresources/conditions/urologic/dvp.aspx. Accessed April 24, 2007.
16. Jadvar H. Therapeutic Solutions Still Lack Comparative Data. Paper presented at: Multidisciplinary Prostate Cancer Symposium, February 2005. Orlando, FL.
17. Prostate Cancer (PDQ): Treatment. Healthcare Professional Overview. National Cancer Institute. Available at: www.cancer.gov/cancertopics/pdq/treatment/prostate/HealthProfessional. Accessed March 31, 2007.
18. Chinn DO. Transrectal HIFU: The next generation? PCRI Insights. 2005;8(1).
19. Kupelian P, Klein E. Overview of Treatment for Early Prostate Cancer. In: Rose BD, ed. UpToDate. Waltham, MA; 2007.
20. Savarese D. Overview of treatment for advanced prostate cancer. In: Rose BD, ed. UpToDate. Waltham, MA; 2007.
21. Lacy CF, Armstrong LL, et al., eds. Drug Information Handbook. 14th ed. Hudson, Ohio: Lexi-Comp; 2006.
22. Arlen PM, Dahut WL, Gulley JL. Immunotherapy for prostate cancer: what's the future? Hematol Oncol Clin North Am. 2006;20:965-83, xi.
23. Arlen PM, Gulley JL, et al. Strategies for the development of PSA-based vaccines for the treatment of advanced prostate cancer. Expert Rev Vaccines. 2003;2:483-493.
24. Sanda MG, DiPaola RS, Simons J, Vieweg J. Vaccine immunotherapies in prostate cancer. A Continuing Medical Education Activity Sponsored by InforMEDical Communications, Inc. 2005. Available at: www2.extendmed.com/capvaccine/p1.html. Accessed May 1, 2007.
25. DiPaola R, Plante M, Kaufman H, et al. A Phase I Trial of Pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in Patients with Prostate Cancer . J Transl Med. 2006;4:1.
26. Phase II study of vaccine therapy comprising vaccinia-PSA-TRICOM vaccine and fowlpox-PSA-TRICOM vaccine combined with sargramostim (GM-CSF) in patients with prostate-specific antigen progression after local therapy for early prostate cancer. National Cancer Institute Web site. Available at: www.cancer.gov/clinicaltrials/ECOG-E9802#AlternateTitle_CDR0000422430). Accessed May 1, 2007.
27. Therion reports results of phase 2 PROSTVAC-VF Trial at ASCO Annual Meeting and formalizes plan for an NCI-sponsored phase 3 study: Potential Survival Difference Identified in Phase 2 Study. Therion Biologics Web site. Available at: www.therionbio.com/news/pressSingle.asp?id=544. Accessed May 1, 2007.
28. Mendiratta P, Armstrong AJ, George DJ. Current standard and investigational approaches to the management of hormone-refractory prostate cancer. Rev Urol. 2007;9(Suppl 1):S9-S19.
29. Gould P. Sipulecel-T shows partial advantage in prostate cancer. Lancet Oncol. 2006;7:710.
30. Sipuleucel-T. Dendreon Web site. Available at: www.dendreon.com. Accessed March 20, 2007.
31. Tarassoff CP, Arlen PM, Gulley JL. Therapeutic vaccines for prostate cancer. Oncologist. 2006;11:451-462.
32. Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089-3094.
33. FDA grants fast-track status to Provenge. Available at: www.bizjournals.com/seattle/stories/2005/11/07/daily3.html. Accessed May 1, 2007.
34. Armstrong AJ, Carducci MA. New drugs in prostate cancer. Curr Opin Urol. 2006;16:138-145.
35. Simons JW, Sacks N. Granulocyte-macrophage colony-stimulating factor–transduced allogeneic cancer cellular immunotherapy: The GVAX vaccine for prostate cancer. Urol Oncol. 2006;24:419-424.
36. GVAX immunotherapy for prostate cancer. Available at: www.cellgenesys.com/clinical-prostate-cancer.shtml. Accessed April 6, 2007.
37. Sonpavde G, Hutson TE. New approaches in hormone refractory prostate cancer. Am J Clin Oncol. 2006;29:196-201.
38. Petrylak DP. The treatment of hormone-refractory prostate cancer: docetaxel and beyond. Rev Urol. 2006;8 Suppl 2:S48-S55.
39. Prostate cancer. The American
Cancer Society. Available at: www.cancer.org/downloads/PRO/ProstateCancer.pdf.
Accessed May 1, 2007.
To comment on this article, contact editor@uspharmacist.com.





