US Pharm. 2006;8:53-64.
Erectile dysfunction (ED) is the inability
to achieve or maintain an erection sufficient for satisfactory sexual
performance.1 ED is estimated to affect up to 30 million American
men. In fact, by the year 2025, it is projected that approximately 322 million
men in industrial nations will be affected by ED, compared with 152 million in
1995.2 The rising number of ED cases is likely due to increased
detection and diagnosis of the condition, particularly after the introduction
of sildenafil (Viagra) to the market. Prevalence rates of ED vary depending
upon the population studied. The Massachusetts Male Aging Study found an
overall prevalence of 52% for any degree of ED in men between ages 40 and 70.
This community-based study found that age was the strongest predictor of ED,
with a 5.1% probability of complete ED at age 40 and a 15% probability at age
70.3 Patients with diabetes, cardiovascular disease, and prostate
disease have also been reported to have an increased prevalence of ED. Current
literature estimates the prevalence of ED in men with diabetes to range from
20% to 71%. However, studies reporting this information do not distinguish
between patients with type 1 versus type 2 diabetes, nor do they control for
disease severity or glycemic control. In general, it estimated that men with
diabetes have a twofold to threefold greater risk for ED compared to men
without diabetes.3,4
Pathophysiology of ED
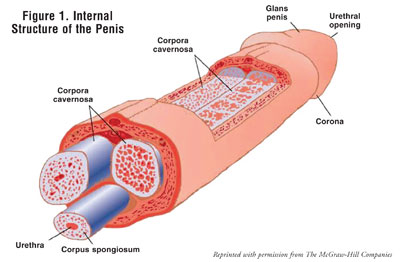
The penis contains two corpora
cavernosa, which are columns of spongelike tissue, connected by sinuses that
become engorged with blood when stimulated (FIGURE 1).5-8 In
the flaccid state, arterial blood flow into the penis is approximately equal
to the venous outflow. When sexually and locally stimulated, nerve impulses
travel from the brain to the vasculature in the penis and lead to an
activation of the parasympathetic nervous system, causing smooth muscle
relaxation and vasodilatation of the penile tissue. This smooth muscle
relaxation increases arterial blood flow into the corpora cavernosa, leading
to elongation, and ultimately, an erection. The maintenance of erections
occurs as a result of compression of the subtunical venules, which decreases
venous outflow from the corpora cavernosa. In addition, acetylcholine
facilitates the production of nitric oxide (NO) from endothelial cells. NO
increases the formation of cyclic guanosine monophosphate (cGMP) to enhance
smooth muscle relaxation and arterial blood flow into the penis.
Once ejaculation has occurred,
smooth muscle tone is increased and blood flow into the corpora cavernosa is
decreased due to the release of norepi? nephrine. Detumescence is further
facilitated by the catabolism of cGMP by phosphodiesterase type 5 (PDE5) to
the inactive metabolite, 5-GMP.5,9
Classification of ED
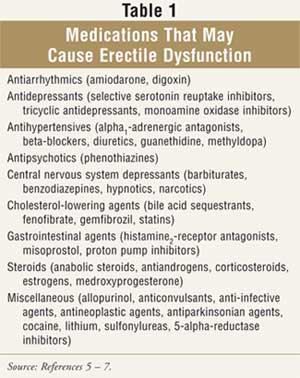
ED may be
classified as organic, psychogenic, or mixed. Organic ED,
the most common type, generally results from a physical cause,
including excessive
alcohol intake, physical inactivity, smoking, inflammatory conditions,
surgical procedures, medications, and chronic diseases (TABLE 1). It is
vital to obtain accurate and complete medication histories, as 10% to 25% of
ED cases are drug induced. Removing or reducing the dose of the causative
agent (e.g., a beta-blocker) may be all that is warranted for the management
of ED. Psychogenic ED is often the result of highly stressful situations and
often manifests only periodically. Mixed ED is the result of both psychogenic
and organic factors.5,6
Diagnosis
When evaluating a
patient with suspected ED, a thorough physical examination is warranted to
exclude prostate and penile abnormalities that might contribute to the
condition. A complete sexual history that includes the frequency, quality, and
duration of erections and presence of morning erections is essential in the
classification of ED. Obtaining a medical history allows the health care
provider to rule out conditions, such as hypogonadism, that may be causing ED.
On the other hand, ED is often the presenting symptom for unidentified
cardiovascular disease.
As discussed earlier, a
medication list of prescription and OTC remedies and illegal drugs allows the
health care provider to determine if the ED is drug induced. Laboratory tests
(e.g., blood glucose levels, lipid profiles, thyroxine levels, testosterone
levels) should be performed to rule out underlying diseases that may cause ED.
Standardized questionnaires, such as the Brief Male Sexual Function Inventory
for Urology and the International Index of Erectile Function, are often used
as screening tools. If a patient's score on a standardized questionnaire
suggests ED, further evaluation is warranted.5,9,10
Management Options
Decision making for
the management of ED should involve both the health care provider and the
patient. Identifying and reversing the cause of ED, if at all possible, is
essential. If a drug is identified as the underlying cause, discontinuation or
dosage reductions may be an option. Underlying diseases should be treated
accordingly, and resolution of ED symptoms should be assessed.
Nonpharmacologic interventions for ED management, other than devices and
surgery, include lifestyle changes such as reduction of stress and/or anxiety,
weight loss, exercise programs, smoking cessation, and moderate alcohol
consumption.5
Phosphodiesterase-Type 5
Inhibitors
The PDE5 inhibitors
are commonly prescribed for the management of ED. As their name indicates,
these agents work to block the type-5–mediated catabolism of cGMP, allowing
NO-induced vasodilatation of the penile vasculature. Sildenafil was the first
PDE5 inhibitor to arrive on the market, in 1998. The usual dose of sildenafil
is 50 mg (25 to 100 mg) taken one hour before sexual activity. The effects of
sildenafil last for approximately four hours, and patients should be
instructed to use no more than one dose within 24 hours. Fatty meals reduce
the absorption of sildenafil; therefore, the drug should be taken on an empty
stomach.5,11
Vardenafil (Levitra), the first second-generation PDE5 inhibitor to be developed, is given at a usual dose of 10 mg (2.5 to 20 mg) one hour before sexual activity. Elderly patients and those with moderate liver dysfunction should receive a lower initial dose of 5 mg. Vardenafil begins working within 30 to 45 minutes after administration and lasts for about four hours. As with sildenafil, patients taking vardenafil should not use more than one dose within a 24-hour period. Patients should not take vardenafil within three hours of fatty meals, due to a reduction in absorption.5,12
The newest PDE5 inhibitor is tadalafil (Cialis), which has a longer duration of action--approximately 36 hours--than sildenafil or vardenafil. In addition, the usual dose of 10 mg (5 to 20 mg) should be taken about 30 minutes before sexual activity--possibly allowing patients more opportunity for spontaneity. Food intake does not appear to affect the absorption of tadalafil; thus, the drug may be taken without regard to meals.5,13
Though considered generally safe for most patients, including those taking multiple antihypertensives, PDE5 inhibitors are not a viable treatment option for every man with ED. Patients with a cardiovascular history that includes a recent myocardial infarction or stroke (within the past two weeks), cerebral vascular accident, life-threatening arrhythmia, hypertension (blood pressure >170/100 mmHg), hypotension (blood pressure <90/50 mmHg), unstable angina, and/or moderate to severe heart failure (New York Heart Association class IIIor IV) should not receive therapy with these agents. The risks and benefits associated with PDE5 inhibitor therapy and the patient's medical history must be assessed. 5,6,11-13
Because PDE5 is inhibited in penile tissue as well as extragenital tissue, patients treated with PDE5 inhibitors may experience headache, facial flushing, nasal congestion, dyspepsia, and dizziness. Sildenafil also inhibits PDE type 6 in the retina. Therefore, patients treated with sildenafil may experience sensitivity to light, blurred vision, and loss of blue-green color discrimination, all of which are generally considered reversible. Tadalafil also inhibits PDE type 11 in skeletal tissue, possibly leading to backand muscle pain. Priapism, or painful, prolonged erections, is an extremely rare adverse effect, especially with shorter-acting agents, such as sildenafil and vardenafil. However, patients should be counseled to seek immediate medical attention if they experience erections lasting longer than four hours. PDE5 inhibitors may also cause various cardiovascular effects, including ventricular arrhythmias, cerebrovascular hemorrhages, myocardial infarctions, transient ischemic attacks, hypertension, and even sudden cardiac death. These adverse cardiac effects confirm the importance of a thorough cardiology evaluation in patients with a significant cardiovascular history.5,6,11-13
PDE5 inhibitors should never be used in patients who are receiving scheduled or intermittent nitrates, due to a risk for severe hypotension. Organic nitrates supply additional NO, which increases cGMP levels and can lead to hypotension. Interestingly, dietary sources of nitrates and nitrites do not interact with the PDE5 inhibitors, as they do not increase circulating levels of NO. The PDE5 inhibitors are metabolized through the cytochrome P-450 isoenzyme 3A4 (CYP3A4). Therefore, inhibitors of CYP3A4 (e.g., cimetidine, ketoconazole, ritonavir) may prolong the effects of PDE5 inhibitors. Patients receiving such agents may need a lower dose of the PDE5 inhibitor. Alpha-adrenergic antagonists (e.g., terazosin, doxazosin, prazosin) commonly used in the management of benign prostatic hyperlasia (BPH)can cause hypotension, especially when given in combination with PDE5 inhibitors. For patients receiving more than 25 mg of sildenafil, the dose of the alpha-antagonist and sildenafil should be separated by at least four hours. Both tadalafil and vardenafil are labeled for precautious use with alpha-antagonists. However, tadalafil may be given concomitantly with tamsulosin 0.4 mg.5,6,11-13
Prostaglandin Analogs
The prostaglandin E
1 analog alprostadil can also be used to treat patients with ED. This
agent mimics endogenous prostaglandin E1 by stimulating adenyl
cyclase to increase the formation of cyclic adenosine monophosphate, thus
increasing arterial blood flow into the corpora cavernosa. Alprostadil is
generally reserved for patients whose ED does not improve with PDE5 inhibitors
and medical devices. Two formulations of alprostadil, a urethral insert (MUSE)
and an intracavernosal injection (Caverject Impulse), are used in the
management of ED and are approved as monotherapy.
MUSE is used via urethral insertion of a pellet containing alprostadil at a dose of 125 to 1,000 mcg about five to 10 minutes before sexual activity. No more than two pellets should be used within a 24-hour period. Patients should be instructed to urinate before insertion of the pellet, as the drug is designed to dissolve in the residual urine in the urethra. Proper use of MUSE is as follows: The cover should be removed from the product, and the applicator should be pushed gently into the urethra using the plunger rod located on top of the insertion device. The device should be rocked gently to ensure the medication has been deposited into the urethra. Once the insertion device has been removed, the penis should be massaged to enhance the absorption of alprostadil. The effects of MUSE last for about 30 to 60 minutes, and the efficacy rate has been reported to range from 43% to 60%. Urethral bleeding and pain are potential adverse effects, especially if the drug is improperly inserted, which may lead to urethral stricture and urinary difficulties. Priapism, dizziness, and syncope have been reported in a small percentage of patients. Female partners of patients receiving MUSE may experience vaginal burning, irritation, pain, or itching due to the transfer of alprostadil during sexual intercourse.5,14,15
Caverject Impulse, an intracavernosal injection of alprostadil, is given at a dose ranging from 2.5 to 60 mcg five to 10 minutes before sexual activity. The dose of Caverject Impulse should be slowly titrated to effect. Patients should not use more than one dose in 24 hours and no more than three doses in a week. The drug is effective for up to one hour. Proper use of Caverject Impulse involves several steps. The dose of the drug is established using a dial on the device. The drug should be injected into only one side of the spongy tissue of the penis, alternating injection sites with subsequent doses. Injections should never be administered on the dorsal or ventral surfaces. Once the drug has been injected, manual pressure should be applied to the site for about five minutes to reduce the risk of hematoma formation. The efficacy rate of Caverject Impulse is much higher than that of MUSE, with 67% to 85% of patients responding to therapy.5,14,16 Intracavernosal alprostadil may be given as a single agent or in combination with vasoactive drugs such as phentolamine and papaverine.1 Intracavernosal injections may be a viable option for patients with diabetes, a population in whom reduced efficacy of PDE5 inhibitors has been seen, as compared to the normal population.17 One possible explanation for the success of intracavernosal therapy in patients with diabetes is that men treated with insulin are accustomed to needles and injections. If the patient with diabetes has peripheral neuropathy, injection site pain may be less than that in a patient who does not have this complication of diabetes.5,14,16,18
The adverse affects associated with Caverject Impulse are mostly local and usually occur during the first year of therapy. Poor injection technique and/or alprostadil itself may cause fibrotic plaques known as corporal fibrosis. Patients who develop these plaques should discontinue therapy with intracavernosal injections, as they are at risk for Peyronie's disease, which makes sexual intercourse even more difficult or potentially impossible. Ten percent to 44% of patients using intracavernosal alprostadil experience penile pain, which typically resolves once the penis is flaccid. However, persistent pain may be present in some men, requiring further treatment and evaluation. Injection-site hematomas are often the result of poor injection technique. To minimize this adverse effect, manual pressure should be applied to the injection site for five minutes. Other adverse effects noted with intracavernosal alprostadil include dizziness and hypotension. Prolonged erections and priapism occurred in 4% and 1%, respectively, of patients who participated in clinical trials. Patients experiencing erections lasting longer than four hours should be advised to seek immediate medical attention, as priapism is a urologic emergency. Patients predisposed to priapism, including those with sickle cell anemia, multiple myeloma, leukemia, polycythemia, or thrombocythemia, should not be treated with any product containing alprostadil.5,14,16
Other Therapies
Treatment of ED
with exogenous testosterone replacement is indicated only for men with
hypogonadism. By replacing endogenous concentrations of testosterone, androgen
receptors are stimulated to maintain libido. ED is not corrected directly
through use of testosterone replacement therapies. Testosterone is available
in multiple formulations, including tablets, gels, intramuscular injections,
and scrotal and dermal patches, allowing treatment to be tailored to meet the
needs of the patient. Before using testosterone, men should undergo screening
for BPH and prostate cancer, as androgen therapies can worsen these
conditions. The adverse effects of testosterone therapy (e.g., weight gain,
acne, exacerbation of hypertension, gynecomastia, edema) are numerous and
common.5
Apomorphine (Uprima) is a dopamine receptor agonist that works to increase erections through stimulation of dopamine2 receptors in the hypothalamus and the limbic system. Five percent to 15% of patients treated with subcutaneous apomorphine for Parkinson's disease experienced frequent erections, spurring further study of a sublingual (SL) formulation not yet FDA approved for ED treatment. Studies have demonstrated that 2 to 3 mg of apomorphine SL is most effective in treating ED, and higher doses show no additional effects. Apomorphine SL has an onset of action of approximately 18 to 20 minutes. The most common adverse effect reported is nausea.19
Yohimbine is an alpha2 -adrenergic antagonist that increases catecholamines to ultimately improve mood. Its proerectogenic properties are believed to allow vasodilatation. Current clinical trials assessing the effects of yohimbine involve small study populations and a short duration of therapy. Increases in blood pressure and heart rate, anxiety, palpitations, and tremors are adverse effects associated with yohimbine.
Papaverine is a smooth muscle relaxant that is often used in combination with phentolamine, an alpha1 -adrenergic antagonist, making smaller doses of each individual component necessary to achieve therapeutic effects. Smaller doses of these agents minimize adverse effects, which include hypotension, hepatotoxicity, and priapism.5,14
Ginseng is often classified as an adaptogen, or an herb that increases resistance to environmental stressors. It is believed to improve stamina and perhaps sexual function. However, data supporting the use of ginseng to treat sexual impairment are limited. Ginseng may cause increased blood pressure, headache, insomnia, pruritus, increased bleeding, and gastrointestinal upset.20,21
Medical Devices
Vacuum erection
devices (VEDs) are safe, viable options for older patients who are more likely
to experience drug-drug and drug-disease interactions with ED pharmacotherapy,
due to higher incidence of comorbidities and number of medications. VEDs may
also benefit patients with ED who do not respond to oral or injectable
therapy. The devices contain three parts: a cylinder in which the flaccid
penis is placed and pushed up flush against the lower abdomen, a pump that
generates a negative pressure to draw blood into the corpora cavernosa, and an
elastic band that is placed at the base of the penis to maintain the erection.
Patients using VEDs have overall satisfaction rates ranging from 60% to 80%;
however, common complaints from patients using these devices include bluish
coloring of the penis; cold feeling of the penis; lack of spontaneity, as the
device may take as long as 30 minutes to achieve an erection; and minor pain
or discomfort with ejaculation.5,22
Surgical Options
Malleable
(noninflatable) and inflatable penile prostheses or implants are available for
the treatment of ED. They are typically reserved for patients who do not
respond to or are not candidates for oral or injectable therapies or VEDs. A
malleable prosthesis, or semirigid rod prosthesis, involves two
bendable rods that are inserted into the corpora cavernosa, allowing the
patient to bend the penis into position prior to intercourse. Patients will
often complain of the appearance of a permanent erection. An inflatable
prosthesis allows for more "natural" flaccidity and erection, as it consists
of two inflatable rods placed into the corpora cavernosa that are connected to
a pump-reservoir. When activated, the device will pump saline from the
reservoir into the inflatable rods, leading to an erection.
The success rate with penile prostheses has been reported to be as high as 98%. However, these devices must be carefully inserted by a urologist during surgery that typically requires general anesthesia. Infection is the most common adverse effect experienced in the acute period following surgery. Patients are still prone to late-onset infections and mechanical failure, although the current five-year failure rate for inflatable prostheses ranges from 6% to 16%, due to improved technology.
Insertion of a penile prosthesis is an expensive procedure that may cause irreversible damage to the penis due to its invasive nature. Therefore, it is often considered a last option for patients with ED.1,5,8
Some patients with ED may be managed through vascular surgery. American Urological Association recommendations recognize arterial reconstructive surgery as an option for patients who have an arterial occlusion with no evidence of generalized vascular disease. Similar to insertion of penile prostheses, vascular surgery carries a risk for infection and damage to the penile tissue.1 Health care providers should assist patients in weighing the risks and benefits associated with the surgical management of ED.
Identification of Barriers to ED
Treatment
Due to the intimate
nature of ED, it is not surprising that several barriers impede patients from
seeking and receiving adequate treatment for the condition. One common barrier
to seeking treatment is embarrassment and/or reluctance to discuss ED with a
health care provider. In particular, some patients may be reluctant to discuss
sexual matters with a female health care provider. The health care provider
may also feel embarrassed. In addition, lack of education about ED, the belief
that ED is a normal part of aging, and the belief that nothing can be done to
adequately treat the condition may impede the patient from receiving care.
However, increased advertising has helped to educate patients on ED treatment
options. Many times, patients fear the adverse effects of the various
treatment options. Cultural differences, language barriers, and the social
stigma regarding sex may cause ED to remain untreated in some patient
populations. Some patients do not want to "waste" the time of the health care
provider by discussing matters that may be considered trivial. Lastly, lack of
affordable treatment options remains one of the greatest barriers to adequate
treatment.23-26
Health care providers need to properly educate patients regarding ED and its various treatment options. Patients should be encouraged to choose a health care provider, either a man or woman, who makes them feel comfortable. Health care providers should routinely ask patients with diabetes, cardiovascular disease, prostate disease, and other risk factors for sexual impairment about their level of sexual satisfaction. A strong provider-patient relationship encourages and facilitates discussion of intimate matters such as ED. Patients should be involved in treatment decisions to increase compliance and satisfaction. Private patient counseling areas should be used to maintain patient confidentiality, minimize embarrassment, and encourage patient discussion. If available, drug samples may be used temporarily to assess tolerability, particularly in patients with financial constraints, although drug sampling is not a long-term resolution.26
The Pharmacist's Role in
Counseling Patients
Pharmacists have a
vital role in the management of patients with ED. Development of a trusting
relationship with the patient facilitates conversation about ED and its
therapies. Based on these discussions, pharmacists can refer patients to
physicians for treatment. Screening for drug-drug interactions (e.g., nitrates
and alpha1-antagonists) and disease-drug interactions may minimize
inappropriate prescribing. Pharmacists must also counsel patients on common
and severe adverse effects of various treatment options, emphasizing the need
to seek immediate medical attention for erections lasting longer than four
hours. Pharmacists may assist in the appropriate treatment management by
suggesting another PDE5 inhibitor for patients experiencing back and/or muscle
pain with tadalafil or blue-green color discrimination difficulties with
sildenafil. Pharmacist collaboration with other health care providers and the
patient allows for optimal management of ED.
REFERENCES
1. Montague DK,
Jarow JP, Broderick GA, et al. Chapter 1: The management of erectile
dysfunction: an AUA update. J Urol. 2005;174:230-239.
2. Sommer F, Engelmann
U. Future options for combination therapy in the management of erectile
dysfunction in older men. Drugs Aging. 2004;21:555-564.
3. Feldman HA,
Goldstein I, Hatzichristou DG, et al. Impotence and its medical and
psychosocial correlates: results of the Massachusetts Male Aging Study. J
Urol. 1994;151:54-61.
4. Bacon CG, Hu FB,
Giovannucci E, et al. Association of type and duration of diabetes with
erectile dysfunction in a large cohort of men. Diabetes Care.
2002;25:1458-1463.
5. DiPiro JT, Talbert
RL, Yee GC, eds. Pharmacotherapy: A Pathophysiologic Approach. New
York: McGraw-Hill; 2005:1515-1533.
6. Setter SM, Iltz JL,
Fincham JE, et al. Phosphodiesterase 5 inhibitors for erectile dysfunction.
Ann Pharmacother. 2005;39:1286-1295.
7. Viera AJ, Clenney
TL, Shenenberger DW, Green GF. Newer pharmacologic alternatives for erectile
dysfunction. Am Fam Physician. 1999;60:1159-1172.
8. Hyde J, DeLamater J,
eds. Internal structure of the penis. In: Understanding Human Sexuality
. New York: McGraw-Hill; 1997.
9. Korenman SG. New
insights into erectile dysfunction: a practical approach. Am J Med.
1998;105:135-144.
10. Keene LC, Davies
PH. Drug-related erectile dysfunction. Adverse Drug React Toxicol Rev.
1999;18:5-24.
11. Viagra [package
insert]. New York, NY: Pfizer; 2005.
12. Levitra [package
insert]. Research Triangle Park, NC: GlaxoSmithKline; 2005.
13. Cialis [package
insert]. Indianapolis, Ind: Eli Lilly; 2005.
14. Seftel AD. From
aspiration to achievement: assessment and noninvasive treatment of erectile
dysfunction in aging men. J Am Geriatr Soc. 2005;53:119-130.
15. MUSE [package
insert]. Mountain View, Calif: Vivus; 2003.
16. Caverject Impulse
[package insert]. Kalamazoo, Mich: Pharmacia & Upjohn: 2003.
17. Perimenis P, Markou
S, Gyftopoulos K, et al. Switching from long-term treatment with
self-injections to oral sildenafil in diabetic patients with severe erectile
dysfunction. Eur Urol. 2002;41:387-391.
18. Penson DF, Wessells
H. Erectile dysfunction in diabetic patients. Diabetes Spectr.
2004;17:225-230.
19. Apomorphine.
DRUGDEX Evaluations. Available at: www.thomsonhc.com. Accessed April 8, 2006.
20. Ginseng, Panax.
Natural Medicines Comprehensive Database. Available at:
www.naturalmedicinesdatabase.com. Accessed April 8, 2006.
21. Hong B, Ji YH, Hong
JH, et al. A double-blind crossover study evaluating the efficacy of Korean
red ginseng in patients with erectile dysfunction: a preliminary report. J
Urol. 2002;168:2070-2073.
22. Montague DK.
Nonpharmacologic treatment of erectile dysfunction. Rev Urol.
2002;4(S3):S9-S16.
23. Shabsigh R,
Perelman MA, Laumann EO, Lockhart DC. Drivers and barriers to seeking
treatment for erectile dysfunction: a comparison of six countries. BJU Int
. 2004;94:1055-1065.
24. Tan NC, Ng CJ, Low
WY, Choo WY. What are the barriers faced by doctors in the management of
erectile dysfunction in general practice? Asia Pac Fam Med. 2004;4:1-6.
25. Perelman M,
Shabsigh R, Seftel A, et al. Attitudes of men with erectile dysfunction: a
cross-national survey. J Sex Med. 2005;2:397-406.
26. Humphery S,
Nazareth I. GPs' views on their management of sexual dysfunction. Fam
Pract. 2001;18:516-518.
To comment on this article, contact editor@uspharmacist.com.






