US Pharm. 2011;36(1):26-42.
Alzheimer's disease (AD) is an irreversible, age-related neurodegenerative disorder defined by a gradual decline in understanding, memory, and ability to perform activities of daily living (ADL). It is the most prevalent type of dementia, accounting for 60% to 80% of all cases.1 Although no medications are available that can reverse the progress of AD, a number of drugs have limited utility in treating cognitive symptoms.
Epidemiology
The prevalence of dementia increases with age, affecting approximately 5% to 8% of people over age 65 years, 15% to 20% of those over age 75 years, and 25% to 50% of those over age 85 years.2 Approximately 5.3 million Americans are affected by AD.1 In the upcoming decades, the baby boom population is estimated to add another 10 million to the number of AD patients diagnosed, resulting in a projected prevalence in 2050 of between 11 million and 16 million people.1 Total annual costs are anticipated to increase from $172 billion in 2010 to $1.08 trillion in 2050.1
Pathophysiology
The exact pathophysiology of AD remains unknown. Classic neuropathologic findings include amyloid plaques, neurofibrillary tangles, and cell death. Beta amyloid-containing plaques are thought to interfere with neuronal activity by increasing free radical production, thereby resulting in oxidative damage to the cells and eventually causing neuronal cell death.3,4
Neurofibrillary tangles are composed of tau protein, which is necessary for the growth and development of strong neuronal axons. When tau proteins are hyperphosphorylated, they form tangles that deposit within neurons in the hippocampus and the medial temporal lobe, making it difficult for neurons to effectively pass signals throughout the cells in the brain. This leads to impaired cognition and memory.3,4
Areas of neuronal cell death and synapse loss are found throughout the brain in a distribution similar to that of neurofibrillary tangles. The death of cholinergic neurons in the basal forebrain interferes with neurotransmitter pathways, leading to a deficit in acetylcholine, a major transmitter involved in memory. Excess glutamate levels in the cerebral cortex also may contribute to learning and memory deficits in AD.3,5
Diagnosis
AD has an insidious onset and gradual progression. According to DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th ed.) criteria, AD involves the development of multiple cognitive deficits, including memory impairment and at least one of the following: aphasia (speech impairment); apraxia (inability to perform previously learned tasks or movements); agnosia (inability to recognize objects or people); and disturbance in executive functioning (tasks requiring multiple steps, such as balancing a checkbook or preparing a meal). This loss in cognitive function causes significant impairment in social or occupational functioning and represents a significant decline from a previous level of functioning.6
The proper diagnosis of AD should be made only when the patient has typical symptoms of the disease and other causes have been ruled out. A definitive diagnosis cannot be made until microscopic examination of the brain at autopsy reveals the characteristic beta amyloid plaques and neurofibrillary tangles. However, a careful clinical diagnosis of AD corresponds to pathologic diagnosis 70% to 90% of the time.2,6
Neuroimaging plays an important role in the differential diagnosis of AD by excluding other causes of dementia. Patients should undergo structural imaging of the brain with CT or MRI at least once in the course of the disease or as part of the initial evaluation. These imaging tests show certain structural and functional changes in the brain that are characteristic of AD. CT shows a reduction in the brain's size, and MRI is beneficial for ruling out other causes of dementia, such as tumor or stroke.3,7 Functional imaging with positron emission tomography or single-photon emission CT also may be helpful in the differential diagnosis of dementia disorders and is approved by Medicare for use in AD diagnosis.3
Genes have been identified that are involved in a variety of dementia syndromes. Most notable of these is apolipoprotein E4, which is a gene on chromosome 19 that is more common in individuals with AD. However, since testing for certain genes is not specific enough to add substantially to diagnostic confidence, it is not currently recommended.3
Progression
The progression of AD is gradual, with an average of 3 to 9 years from symptom onset to death.8 Deterioration--including that of behavior, cognition, and daily function--occurs across many domains as the disease progresses, but symptoms vary between patients and between stages.5 The decline may plateau at times, but progression generally resumes within one to several years.2 Neuropsychiatric symptoms such as depression, anxiety, irritability, aggression, and personality changes are common in the early stages of AD, with psychotic symptoms and behavioral disturbances noted in the middle and later stages.2 Because of the variability and progression of AD, treatment targets should be revisited and revised as the disease progresses in order to address newly emerging issues.
The Mini-Mental State Examination (MMSE) is a tool often used in practice to determine baseline cognitive function and measure changes in AD severity. MMSE scores range from 0 to 30 points, with higher scores indicating less cognitive impairment. The MMSE measures attention, orientation, registration, recall, language, and ability to copy a figure.9
According to American Psychiatric Association guidelines, mild AD (MMSE score >18) is marked by forgetfulness and difficulty performing simple activities, such as driving and balancing a checkbook.2 Moderate AD (MMSE score 10-18) involves noticeable memory loss and an increased need for assistance with ADL. This stage also may be marked by wandering, insomnia, and delusions. Severe AD (MMSE score <10) is characterized by a need for total assistance with personal care. Patients in this stage may also demonstrate agitation, incontinence, and limited language ability.2
People with dementia are two to four times more likely to die at a given age than those of the same age without dementia.8 In itself, AD is not a cause of death, but as the disease progresses, conditions often develop that may lead to death. These include sepsis, upper respiratory infections, malnutrition disorders, fractures, and wounds. The management and proper identification of these conditions is critical in caring for the patient with AD.8,10
Pharmacotherapy
At present, no mechanisms exist for slowing, reversing, or stopping the pathologic processes of AD. Therefore, the main goals of therapy are to minimize behavioral disturbances and improve symptoms.5 Currently, five drugs are FDA approved for managing AD (TABLE 1). Four of these--donepezil, galantamine, rivastigmine, and tacrine--are cholinesterase inhibitors (ChEIs). The fifth agent is memantine, a noncompetitive N-methyl-d-aspartic acid (NMDA) receptor antagonist.9
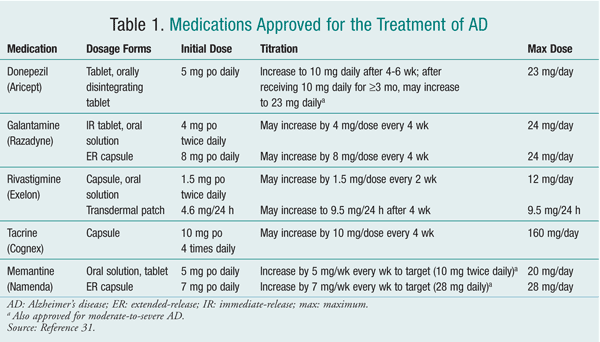
ChEIs: ChEIs work by degrading acetylcholinesterase, thereby causing an increase in levels of acetylcholine, a neurotransmitter important in cognition and memory. Tacrine, the first ChEI approved for use in AD (1993), is associated with reversible, direct medication-induced hepatotoxicity occurring in approximately 30% of patients. Liver-function tests are required every other week from weeks 4 through 16 and at least every 3 months thereafter. Because of its side effects, required extensive monitoring, and multiple daily dosing, tacrine is rarely used in practice now.2
Studies have repeatedly found an advantage of galantamine, rivastigmine, and donepezil over placebo in both cognitive and functional measures, thus decreasing caregiver burden and delaying time to nursing home placement. The magnitude of benefit is similar for all three agents.5 Therefore, these three drugs are considered to be first-line agents. Treatment with a ChEI should be initiated upon diagnosis of AD.
All of these agents are approved for mild-to-moderate AD; however, only donepezil carries an indication for moderate-to-severe AD.2 In July 2010, the FDA approved an increased donepezil dosage for patients with moderate-to-severe AD not improving on the previous maximum dosage (10 mg daily).11 Approval was based on an international study by Farlow and colleagues that examined the effectiveness and tolerability of high-dose (23 mg daily) versus standard-dose (10 mg daily) donepezil in moderate-to-severe AD. 12 The 23-mg dosage demonstrated a statistically significant improvement in cognition, but there was no significant difference in global function (patient function as defined by clinician) versus the 10-mg dosage.
ChEIs exhibit a dose-response relationship. Therefore, patients should be titrated to the maximum dose tolerated. If intolerable adverse events (AEs) occur with ChEIs, treatment should be discontinued for at least 1 week or until AEs resolve.2
The most commonly noted AEs (affecting about 10% to 20% of patients) with ChEIs are nausea and vomiting, which are usually mild to moderate in severity. In most cases, the nausea and vomiting diminish within 2 to 4 days. Other AEs include muscle cramps, bradycardia, increased gastrointestinal (GI) acid, reduced appetite, and decreased weight.
Gill and colleagues noted that AEs of syncope and bradycardia associated with ChEIs may be clinically underrecognized, leading to such serious consequences as falls, hip fractures, or permanent pacemaker implantation.13 Patients taking ChEIs should be monitored for these AEs, and a trial discontinuation of the ChEI should be considered prior to permanent pacemaker implantation.13 Bradycardia and hypersensitivity to the individual drug should be considered relative contraindications to ChEI use. Precautions for treatment with ChEIs are GI disorders (e.g., gastritis, ulcerative disease, undiagnosed nausea and vomiting); sick sinus syndrome (arrhythmias caused by sinus node dysfunction); conduction defects; cerebrovascular disease; seizures; asthma; or chronic obstructive pulmonary disease. ChEIs may exacerbate urinary obstruction or sleep disturbances or exaggerate the effects of some muscle relaxants during anesthesia.2
Rivastigmine should be given with food to minimize nausea. Rivastigmine is also available as a transdermal patch, which may benefit patients experiencing a high degree of nausea or vomiting with oral ChEIs or those with low adherence rates.14 Guidelines for switching patients from oral to transdermal ChEIs are not clear. Sadowsky and colleagues suggested that patients taking high-dose rivastigmine (>6 mg/day) may be switched directly to the 9.5-mg/24-hour patch, whereas patients taking a lower dosage (<6 mg/day) should be started on the 4.6-mg/24-hour patch. It also was suggested to start patients on the 4.6-mg/24-hour patch when switching from donepezil or galantamine.14 Donepezil is available as an orally disintegrating tablet, which may be helpful for patients with swallowing disorders that can occur in later-stage AD.1
Results of studies comparing the safety, tolerability, and efficacy of the different ChEIs vary, but a pattern of fewer AEs in patients treated with donepezil versus rivastigmine or galantamine has been noted.15 One trial also found that rivastigmine alone or in combination with memantine may be effective in patients who fail donepezil or galantamine treatment.16
There is no clear evidence indicating how long to treat patients with ChEIs. Data from clinical trials have demonstrated benefits over placebo for up to 2 years. The decision to continue treatment with ChEIs beyond proven efficacy should be individualized, as clinical trials of long-term continuation have not been conducted. Reasons to discontinue treatment include AEs, lack of motivation or compliance, lack of perceived efficacy, and cost.
A physician may consider continuing ChEIs if the patient's symptoms have stabilized or decline has slowed and he or she wishes to continue treatment. A patient observed to be declining rapidly despite treatment with a ChEI should be considered a medication nonresponder. In this case, the ChEI may be stopped or a different ChEI may be tried.2,16,17 In placebo-controlled trials, discontinuation of ChEIs has caused deterioration of cognitive improvement to the level of the corresponding placebo group. It is unclear whether restarting ChEIs can reverse the worsening that occurs with treatment interruption. Doody and colleagues found that donepezil-treated patients whose treatment was suspended for 6 weeks never regained cognition to the level achieved before medication discontinuation.18
NMDA Receptor Antagonist: Memantine works by partially blocking the NMDA receptor to prevent excess stimulation of the glutamate system. Glutamate may contribute to the destruction of cholinergic neurons through neuronal calcium overload. Memantine may prevent neurotoxicity without interfering with the role of glutamate in learning and memory.19 Results of studies comparing ChEI monotherapy with ChEI plus memantine have found that patients receving combination therapy had significantly better measures of cognitive function and ADL.20,21 Given the evidence for its safety and efficacy in randomized, controlled trials, memantine should be considered for patients with moderate-to-severe AD.2,19 AEs with memantine are infrequent and mild, but include nausea, confusion, dizziness, headache, diarrhea, sedation, agitation, falls, and constipation.2,9,19
In June 2010, the FDA approved an extended-release (ER) formulation of memantine (Namenda XR) with once-daily dosing.11 Approval was based on a 24-week, randomized, double-blind, placebo-controlled trial involving 677 patients with moderate-to-severe AD receiving a stable dose of a ChEI. The addition of memantine ER to ChEI therapy resulted in significant improvement in cognitive function versus treatment with ChEI and placebo.22 Currently, no trials are assessing the comparative efficacy of memantine immediate-release versus ER or examining a switch between dosage forms. The manufacturer states that patients taking memantine 10 mg twice daily may be switched to memantine ER 28 mg once daily starting the day following the last 10-mg tablet.22
Other Agents: Several other medications have been proposed for the treatment of AD or the prevention of cognitive decline. A few epidemiologic studies found that indomethacin therapy may lower the risk of AD, although a number of larger studies with various nonsteroidal anti-inflammatory drugs (NSAIDs) demonstrated no benefit over placebo.23,24 NSAIDs also increase the risk of GI, renal, and cardiac toxicities.23 The Lipitor's Effect in Alzheimer's Dementia study, which examined the use of the hydroxymethyl glutaryl coenzyme A reductase inhibitor atorvastatin, failed to show significant clinical benefit in cognition or functioning compared with placebo and donepezil.25
Herbal products, especially ginkgo biloba, have been extensively studied for use in dementia. Data for gingko are inconclusive, and the herb has not been proven to provide any benefit for AD.26 Epidemiologic studies proposed that vitamin E may delay the onset of AD, but recent studies reported that it is associated with a small but significantly increased mortality risk and an increased rate of heart failure.27,28 Case studies have proposed that hormone therapy with estrogen may help decrease the risk of dementia.2 However, a large prospective study found that estrogen actually increased the risk of developing AD.29 None of the above therapies has conclusive or consistent data supporting its use in the treatment or prevention of AD.
Future Directions
AD is one of the most significant health crises facing the United States in the upcoming decades.30 Researchers continue to study many aspects of AD, including identifying risks for developing the disease; methods for better detection and earlier diagnosis; and ways to prevent, slow, or cure AD. A number of medications are being studied, some of which show promise in phase II and III trials. Research is focusing on medications that can prevent plaque deposition, remove plaque or amyloid fragments, prevent formation of neurofibrillary tangles, or promote the breakup of tangles in the brain.1
Pharmacist Involvement With Patients and Caregivers
While pharmacists interact with the patient in early-stage AD, the primary source of interaction shifts to the caregiver as the disease progresses and the patient's cognitive function declines. Caregiver distress is a major concern in the treatment of AD. Although caregivers are often proud of the help they provide, they may become frustrated, overwhelmed, or depressed, which can result in poor health outcomes for both caregiver and patient.1,2 This can lead to an increased risk of substandard care, neglect, or abuse of patients.
Caregivers must be aware of the demands that may be made on them and find ways to take care of themselves as well.2 Signs of caregiver distress include anger, anxiety, exhaustion, insomnia, irritability, poor concentration, social withdrawal, health problems, and denial. Caregivers should be encouraged to take proper care of their spiritual, emotional, and physical health and to seek support when needed.2 Pharmacists are ideally suited to identify caregiver distress and to offer support and information to improve outcomes for both patient and caregiver (see RESOURCES Sidebar).
Health care professionals do not always consider caregivers' position or the burdens placed on them. Because pharmacists are trusted and respected, their availability enables them to participate in the care of both patient and caregiver in many ways. Increased involvement with AD patients may improve the patient's clinical outcome and the caregiver's quality of life. Early medication management should be encouraged, and the pharmacist should review all medications for AEs, appropriate dosing, and drug interactions. The pharmacist should check for medications with anticholinergic properties and consider recommending alternative treatments, since these agents can negate the potential beneficial effects of ChEIs.
It is important to communicate with both patient and caregiver concerning all aspects of the medications, including proper administration and possible AEs (to help maintain adherence). The pharmacist should work with the patient and caregiver to identify and reduce any existing medication issues, such as AEs or adherence problems.30
Although most caregivers work diligently to help patients through this long and debilitating illness, pharmacists should be aware that physical, emotional, and financial abuse of afflicted patients does sometimes occur. If such a situation is suspected, pharmacists should report their suspicions. In many states, the reporting of such abuse is mandatory.
For caregivers, pharmacists can encourage early intervention and planning (e.g., setting up power of attorney and advance directives at an early stage in the disease); provide ongoing support and education; and help identify realistic expectations about AD and its progression and management.30 Referrals to local and national support groups can help relieve caregiver distress.2 Pharmacists should also advise patients, families, and caregivers to contact their local Alzheimer's Disease Association office for additional information and support. Given the anticipated increase in the number of people suffering from AD, resources, services, and support will be in greater demand, and pharmacists are in a perfect position to help meet this demand.30
REFERENCES
1. Alzheimer's Association. www.alz.org/index.asp. Accessed April 30, 2010.
2. Work Group on Alzheimer's Disease and Other Dementias. Practice Guideline for the Treatment of Patients with Alzheimer's Disease and Other Dementias. 2nd ed. American Psychiatric Association; 2007. www.psychiatryonline.com/
3. Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143-1153.
4. Näslund J, Haroutunian V, Mohs R, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571-1577.
5. Geldmacher DS. Treatment guidelines for Alzheimer's disease: redefining perceptions in primary care. Prim Care Companion J Clin Psychiatry. 2007;9:113-121.
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
7. Duara R, Loewenstein DA, Potter E, et al. Medial temporal lobe atrophy on MRI scans and the diagnosis of Alzheimer's disease. Neurology. 2008;71:1986-1992.
8. Xie J, Brayne C, Matthews FE, et al. Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. BMJ. 2008;336:258-262.
9. Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379-397.
10. Chandra V, Bharucha NE, Schoenberg BS. Conditions associated with Alzheimer's disease at death: case-control study. Neurology. 1986;36:209-211.
11. Drugs@FDA. Drug approval reports. www.accessdata.fda.gov/
12. Farlow MR, Salloway S, Tariot PN, et al. Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe alzheimer's disease: a 24-week, randomized, double-blind study. Clin Ther. 2010;32:1234-1251.
13. Gill SS, Anderson GM, Fischer HD, et al. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Arch Intern Med. 2009;169:867-873.
14. Sadowsky C, Perez JA, Bouchard RW, et al. Switching from oral cholinesterase inhibitors to the rivastigmine transdermal patch. CNS Neurosci Ther. 2010;16:51-60.
15. Lockhart IA, Mitchell SA, Kelly S. Safety and tolerability of donepezil, rivastigmine and galantamine for patients with Alzheimer's disease: systemic review of the 'real-world' evidence. Dement Geriatr Cogn Disord. 2009;28:389-403.
16. Dantoine T, Auriacombe S, Sarazin M, et al. Rivastigmine monotherapy and combination therapy with memantine in patients with moderately severe Alzheimer's disease who failed to benefit from previous cholinesterase inhibitor treatment. Int J Clin Pract. 2006;60:110-118.
17. Gardette V, Andrieu S, Lapeyre-Mestre M, et al. Predictive factors of discontinuation and switch of cholinesterase inhibitors in community-dwelling patients with Alzheimer's disease: a 2-year prospective, multicentre, cohort study. CNS Drugs. 2010;24:431-442.
18. Doody RS, Geldmacher DS, Gordon B, et al. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol. 2001;58:427-433.
19. Livingston G, Katona C. The place of memantine in the treatment of Alzheimer's disease: a number needed to treat analysis. Int J Geriatr Psychiatry. 2004;19:919-925.
20. Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291:317-324.
21. Atri A, Shaughnessy LW, Locascio JJ, Growdon JH. Long-term course and effectiveness of combination therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:209-221.
22. Namenda XR (memantine hydrochloride) product information. St. Louis, MO: Forest Laboratories; June 2010.
23. Aisen PS, Schafer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819-2826.
24. Martin BK, Szekely C, Brandt J, et al. Cognitive function over time in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896-905.
25. Feldman HH, Doody RS, Kivipelto M, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74:956-964.
26. DeKosky ST, Williamson JD, Fitzpatrick AL, et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300:2253-2262.
27. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46.
28. Lonn E, Bosch J, Yusuf S, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338-1347.
29. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
30. Skelton JB. White paper on expanding the role of pharmacists in caring for individuals with Alzheimer's disease: APhA Foundation Coordinating Council to Improve Collaboration in Supporting Patients with Alzheimer's Disease. J Am Pharm Assoc (2003). 2008;48:715-721.
31. Clinical Pharmacology database. www.clinicalpharmacology-ip.
To comment on this article, contact rdavidson@uspharmacist.com.






