US Pharm. 2016;41(3):55-56.
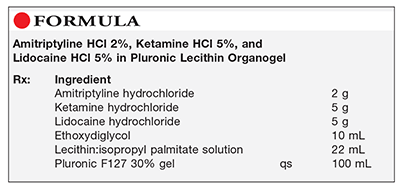
Method of Preparation: Note—The lecithin:isopropyl palmitate solution can be prepared by mixing 0.2 g of sorbic acid, 50 g of soy lecithin, and 50 g of isopropyl palmitate. The Pluronic F127 solution can be prepared by mixing 0.2 g of potassium sorbate, 30 g of Pluronic F127, and sufficient purified water to make 100 mL.
Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh or measure each ingredient. Mix the active ingredients in the ethoxydiglycol until smooth. Incorporate the lecithin:isopropyl palmitate solution and mix well. Add sufficient Pluronic F127 30% gel to volume and mix thoroughly, using a mechanical shearing force. Package and label.
Use: This preparation has been used in the topical treatment of mild-to-moderate pain.
Packaging: Package in tight, light-resistant containers.
Labeling: Use only as directed. Keep out of reach of children. For external use only.
Stability: A beyond-use date of up to 30 days may be used for this preparation.1
Quality Control: Quality-control assessment can include theoretical weight compared with actual weight, pH, specific gravity, active drug assay, color, texture–surface, texture–spatula spread, appearance, feel, rheologic properties, and physical observations.2
Discussion: Amitriptyline hydrochloride (Elavil, C20H23N.HCl, MW 313.86) is a dibenzocycloheptene derivative tricyclic antidepressant. It occurs as a white or practically white, odorless or practically odorless, crystalline powder or small crystals. Amitriptyline hydrochloride is freely soluble in water, alcohol, chloroform, and methanol.1
Ketamine hydrochloride (C13H16ClNO.HCl, MW 274.2) is used as an anesthetic and analgesic. It occurs as a white, crystalline powder with a slight characteristic odor. Ketamine hydrochloride is soluble 1 g in 4 mL of water, 14 mL of alcohol, and 60 mL of absolute alcohol.1,3
Lidocaine HCl (C14H22N2O.HCl.H2O, MW 288.81) occurs as a white, odorless, crystalline powder with a slightly bitter taste. It is an amide-type local anesthetic. It is very soluble in water (1:0.7) and in alcohol (1:1.5).1,2
Ethoxydiglycol (C6H14O3, CH2OHCH2OCH2CH2OC2H5, MW 134.20) is also called diethylene glycol monoethyl ether, diethylene glycol ethyl ether, Carbitol, and Transcutol. It occurs as a colorless liquid with a mild, pleasant odor. Ethoxydiglycol is hygroscopic and is miscible with water and with common organic solvents. It is used as a solvent, solubilizer, and cosurfactant.4
Lecithin (egg lecithin, soybean lecithin, vegetable lecithin) describes a complex mixture of acetone-insoluble phosphatides. It is used as an emollient, emulsifying agent, and solubilizing agent in topicals. Lecithin derived from vegetable sources has a bland or nutlike taste and varies from brown to light yellow, depending upon whether it is bleached or unbleached. Physically, lecithins range from viscous semiliquids to powders. They are practically insoluble in water, polar solvents, and cold vegetable and animal oils; when mixed with water, however, they hydrate to form emulsions. Lecithins are soluble in aliphatic and aromatic hydrocarbons, mineral oil, and fatty acids.5
Isopropyl palmitate (C19H38O2, MW 298.51) is a colorless, mobile liquid with a very slight odor that is used as an emollient, oleaginous vehicle, and solvent; it has good spreading characteristics. Isopropyl palmitate is soluble in acetone, castor oil, cottonseed oil, alcohol, and mineral oil; it is insoluble in water, glycerin, and propylene glycol.6
Pluronic F127 is a block copolymer of ethylene oxide and propylene oxide that is used as an emulsifying agent, solubilizing agent, and wetting agent. It occurs as white-colored, waxy, free-flowing granules or as cast solids that are practically odorless and tasteless. The pH of a 2.5% w/v aqueous solution is in the range of 6.0 to 7.4. Pluronic F127 is freely soluble in water, alcohol, and isopropyl alcohol.7
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; February 2016.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of ointments/creams/gels. IJPC. 1998;2:308-309.
3. Sweetman SC, ed. Martindale: The Complete Drug Reference. 33rd ed. London, England: Pharmaceutical Press; 2002:1262-1263.
4. Ash M, Ash I. Handbook of Pharmaceutical Additives. Brookfield, VT: Gower Publishing Ltd; 1995:484.
5. Shah HC, Sheng JJ. Lecithin. In: Rowe RC, Sheskey PJ, Cook WG, Fenton ME, eds. Handbook of Pharmaceutical Excipients. 7th ed. Washington, DC: American Pharmaceutical Association; 2012:437-439.
6. Taylor AK. Isopropyl palmitate. In: Rowe RC, Sheskey PJ, Cook WG, Fenton ME, eds. Handbook of Pharmaceutical Excipients. 7th ed. Washington, DC: American Pharmaceutical Association; 2012:400-402.
7. Cable CG. Poloxamer. In: Rowe RC, Sheskey PJ, Cook WG, Fenton ME, eds. Handbook of Pharmaceutical Excipients. 7th ed. Washington, DC: American Pharmaceutical Association; 2012:573-577.
To comment on this article, contact rdavidson@uspharmacist.com.





