US Pharm. 2013;8(38):41-42.
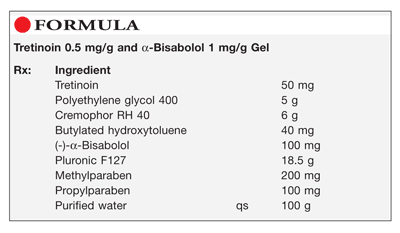
Method of Preparation: Calculate the quantity of each ingredient for the amount to be prepared. Accurately weigh or measure each ingredient. Heat approximately 70 mL of purified water to about 70°C and dissolve the methylparaben and propylparaben; cool to about 40°C. Mix the tretinoin, Cremophor RH 40, butylated hydroxytoluene, and (-)-α-bisabolol in the polyethylene glycol (PEG) 400, heated to about 40°C, until clear. Combine the two solutions. Add approximately 14 g of the Pluronic F127 and mix well; cool to about 6°C, add the remainder of the Pluronic F127, and mix well. Maintain at refrigerated temperature until all the air bubbles have escaped. Add sufficient purified water to final weight and mix well. Package and label.
Use: This preparation is used in the treatment of acne vulgaris, photodamaged skin, fine wrinkles, mottled hyperpigmentation, and tactile roughness of facial skin.
Packaging: Package in tight, light-resistant containers.
Labeling: For external use only. Keep out of reach of children. Protect from light. Discard after [time period] ____.
Stability: A beyond-use date of up to 6 months may be used for this preparation.1
Quality Control: Quality-control assessment may include theoretical weight compared with actual weight, pH, specific gravity, active drug assay, color, clarity, texture-surface, texture-spatula spread, appearance, feel, rheologic properties, and physical observations (clear, yellowish gel).2
Discussion: Tretinoin (C20H28O2, MW 300.44) occurs as a yellow to light-orange, crystalline powder that is insoluble in water and slightly soluble in alcohol. Tretinoin is sensitive to light, heat, and air, especially when it is in solution.3
PEG 400 (Carbowax, polyoxyethylene glycol) occurs as a clear, colorless or slightly yellow-colored, viscous liquid with a slight but characteristic odor and a bitter, slightly burning taste. It is soluble in water and miscible in all ratios with other PEGs. PEG 400 is soluble in acetone, alcohols, glycerin, and glycols and insoluble in fats, fixed oils, and mineral oil.4
About 75% of the components in Cremophor RH 40 (polyoxyl 40 hydrogenated castor oil) are hydrophobic, consisting mainly of fatty acid esters of glycerol PEG and fatty acid esters of PEG. The hydrophilic portion consists of PEGs and glycerol ethoxylates. It is used as an emulsifying agent, solubilizing agent, and wetting agent. It occurs as a white to yellowish, semisolid paste at 20°C that liquefies at 30°C.5
Butylated hydroxytoluene (BHT, C15H24O, MW 220.35) is an antioxidant that occurs as a white or pale yellow, crystalline solid or powder with a faint characteristic odor. It is practically insoluble in water, glycerin, propylene glycol, solutions of alkali hydroxides, and dilute aqueous mineral acids. It is freely soluble in ethanol, methanol, fixed oils, and liquid paraffin.6
(-)-α-Bisabolol (levomenol, C15H26O, MW 222.4) is a sesquiterpene isolated from the volatile oil of chamomile. It is present in many emollient preparations and has been used as a penetration enhancer. It has anti-irritant, anti-inflammatory, and antimicrobial properties.3
Pluronic F127 is used as an emulsifying agent, solubilizing agent, and thickener. It is a white-colored, waxy, free-flowing granule that is practically odorless and tasteless. Pluronic F127 is freely soluble in water, alcohol, and isopropyl alcohol.7
Methylparaben (methyl hydroxybenzoate, methyl parahydroxybenzoate, C8H8O3) is available as colorless crystals or as a white, crystalline powder that is odorless or almost odorless and has a slight burning taste. It is an antimicrobial preservative.8
Propylparaben (propyl hydroxybenzoate, propyl parahydroxybenzoate, C10H12O3) occurs as a white, crystalline, odorless, and tasteless powder. It is an antimicrobial preservative.8
REFERENCES
1. U.S. Pharmacopeia 35/National Formulary 30. Rockville, MD: U.S. Pharmacopeial Convention, Inc; 2012:344-350,350-386.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of ointments/creams/gels. IJPC. 1998;2:308-309.
3. Sweetman SC, ed. Martindale: The Complete Drug Reference. 35th ed. London, England: Pharmaceutical Press; 2007:1455-1456,2116.
4. Wallick D. Polyethylene glycol. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. Washington, DC: American Pharmaceutical Association; 2009:517-522.
5. Singh KK. Polyoxyethylene castor oil derivatives. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. Washington, DC: American Pharmaceutical Association; 2009:542-549.
6. Guest RT. Butylated hydroxytoluene. In: Rowe RC, Sheskey PL, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. Washington, DC: American Pharmaceutical Association; 2009:75-76.
7. Collett JH. Poloxamer. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. Washington, DC: American Pharmaceutical Association; 2009:506-509.
8. Sandler N. Methylparaben and propylparaben. In: Rowe RC, Sheskey PJ, Cook WG, Fenton ME, eds. Handbook of Pharmaceutical Excipients. 7th ed. Washington, DC: American Pharmaceutical Association; 2012:500-504,679-682.
To comment on this article, contact rdavidson@uspharmacist.com.





