US Pharm. 2012;37(1):34-35.
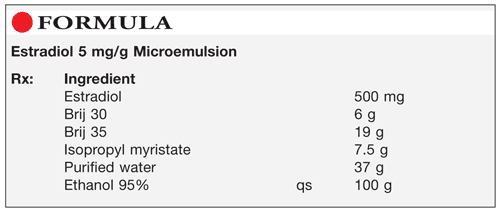
Method of Preparation: Calculate the quantity of each ingredient for the amount to be prepared. Accurately weigh or measure each ingredient. Mix together the Brij 30 and Brij 35. Incorporate the isopropyl myristate, purified water, and ethanol to a weight of about 95 g. Add the estradiol (E2); mix well. Add sufficient ethanol to final weight; mix well. Package and label.
Use: E2 microemulsion is used as a transdermal E2 dosage form.
Packaging: Package in tight, light-resistant containers.
Labeling: Keep out of the reach of children. Apply using gloves. Discard after ____ [time period].
Stability: A beyond-use date of 30 days may be used for this preparation.1
Quality Control: Quality-control assessment may include theoretical weight compared with actual weight, pH, specific gravity (SG), active drug assay, color, clarity, texture–surface, texture–spatula spread, appearance, feel, rheologic properties, and physical observation.2
Discussion: Transdermal hormonal delivery supports patient compliance, reduces first-pass metabolism, avoids gastrointestinal irritation, prolongs delivery, and reduces systemic side effects. This delivery system uses a new microemulsion dosage form.3
E2 is a naturally occurring steroidal estrogen. E2 (beta-estradiol, oestradiol, C18H24O2, MW 272.38) occurs as white or creamy-white, odorless, hygroscopic small crystals or crystalline powder. It is practically insoluble in water, soluble 1 g in 28 mL alcohol, and sparingly soluble in vegetable oils. It should be stored in airtight, light-resistant containers at controlled room temperature and protected from light.
In the body, E2 is reversibly oxidized to estrone, and both E2 and estrone can be converted to estriol. Generally, E2 is not used orally because of extensive first-pass hepatic metabolism. E2 is indicated in the treatment of atrophic vaginitis, atrophic vulval dystrophy, menopausal symptoms, female hypogonadism, ovariectomy, primary ovarian failure, mild-to-severe vasomotor symptoms associated with menopause, inoperable breast cancer, and inoperable prostate cancer.4-6
Brij 30 (polyoxyl-4 lauryl ether, laureth-4, Lipocol L-4) and Brij 35 (polyoxyl-23 lauryl ether, laureth-23, Lipocol L-23) are polyoxyethylene alkyl ethers that serve as emulsifying agents, penetration enhancers, solubilizing agents, and wetting agents. Brij 30 is a colorless to pale yellow liquid with a melting point of about 2°C. Brij 35 is a white, waxy solid with a melting point of about 33°C. Brij 30 is soluble in ethanol, propylene glycol (PG), and water and is dispersible in fixed oils and mineral oil. Brij 35 is soluble in ethanol, PG, and water and is insoluble in fixed oils and mineral oil. The antimicrobial efficacy of the parabens may be reduced owing to hydrogen bonding.7
Isopropyl myristate (C17H34O2, MW 270.51) is composed of esters of propan-2-ol and saturated high-molecular-weight fatty acids, principally myristic acid. It is used as an emollient, solvent, skin penetrant, and oleaginous vehicle. It is absorbed readily by the skin and is used as a component of semisolid bases in pharmaceutical and cosmetic formulations. Isopropyl myristate is a clear, colorless, practically odorless mobile liquid with a bland taste. It has an SG of 0.846 to 0.854, a boiling point of 140.2°C, and a freezing point of about 3°C. It is miscible with acetone, chloroform, ethanol, ethyl acetate, fats, fatty alcohols, fixed oils, liquid hydrocarbons, and waxes and is practically insoluble in glycerin, PG, and water. Isopropyl myristate is resistant to oxidation and hydrolysis and does not become rancid, but it should be stored in a well-closed container in a cool, dry place protected from light. Listed incompatibilities include contact with rubber and plastics, which causes these to swell; mixing isopropyl myristate with hard paraffin causes a granular mixture, and it should not be mixed with strong oxidizing agents.8
Alcohol (ethyl alcohol, ethanol, grain alcohol, C2H5OH, MW 46.07) is a clear, colorless mobile and volatile liquid with a slight characteristic odor and a burning taste. Its SG is between 0.812 and 0.816; its boiling point is 78.15°C. Alcohol is miscible with glycerin and water and should be stored in a cool place. It is incompatible with oxidizing materials in acidic conditions. With alkalies, it may darken in color. When it is added to aqueous solutions of organic salts or acacia, these may precipitate. Alcohol is incompatible with aluminum containers, and it may interact with some drugs.9
REFERENCES
1. USP Pharmacists’ Pharmacopeia. 2nd ed. Rockville, MD: US Pharmacopeial Convention, Inc; 2008:775-779,797-831.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of ointments/creams/gels. IJPC. 1998;2:308-309.
3. Tsai YH, Fu LT, Huang CT, et al. Formulation optimization of estradiol microemulsion using response surface methodology. J Pharm Sci. 2011;100:4383-4389.
4. McEvoy GK. AHFS Drug Information 2011. Bethesda, MD: American Society of Health-System Pharmacists; 2011:3133-3138.
5. Sweetman SC, ed. Martindale: The Complete Drug Reference. 37th ed. London, England: Pharmaceutical Press; 2011:2303.
6. Lacy CF, Armstrong LL, Goldman MP, Lance LL. Drug Information Handbook. 13th ed. Hudson, OH: Lexi-Comp, Inc; 2005:549-553.
7. Gupta RT, Singh KK. Polyoxyethylene alkyl ethers. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:536-542.
8. Taylor AK. Isopropyl myristate. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:348-349.
9. Quinn ME. Alcohol. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:17-19.
To comment on this article, contact rdavidson@uspharmacist.com.






