U.S. Pharm. 2015;40(12):HS36-HS40.
Delayed gastric emptying, also known as gastroparesis, is a disorder that either slows or stops movement of food through the gastrointestinal (GI) tract. It commonly causes nausea, vomiting, heartburn, erratic blood glucose levels, and postprandial fullness.1 Normally, the smooth muscles of the GI tract move food down to the stomach and through the intestines, where it is excreted through the rectum. This process is controlled by the vagus nerve, a component of the parasympathetic nervous system. The most common causes of gastroparesis are diabetes mellitus and surgery; it may also be of unknown cause. Diabetes accounts for almost one-third of cases of gastroparesis, with about 5% to 12% of all diabetes patients experiencing this disorder.2,3 Diagnosis is most often determined by performing endoscopy or using scintigraphy—a specialized radiologic imaging technique—to measure the rate at which radioactive food passes through the GI tract. The Gastric Emptying Breath Test (GEBT) makes it possible to diagnose gastroparesis without the use of radioactive materials.Gastric Emptying Breath Test
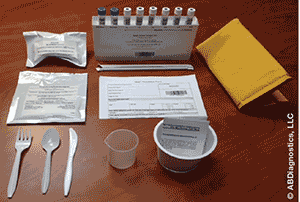
Developed by Advanced Breath Diagnostics, the GEBT is a non-radioactive test that utilizes carbon-13 (13C). Labeling with 13C stable isotope is essentially safe, as 1.1% of our bodies, and of the food we eat, consists of 13C; the remaining 98.9% consists of 12C.4 The GEBT test was developed for adult patients who are symptomatic for gastroparesis. The test measures the rate of gastric emptying of solids and aids in the diagnosis of gastroparesis. The test system utilizes a gas isotope ratio mass spectrometer for the measurement of the ratio of 13CO2 to 12CO2 in breath samples. Administration of the GEBT does not require a special facility, but it should be administered under the guidance of a healthcare professional.4 The GEBT is shown in FIGURE 1.
Test Administration
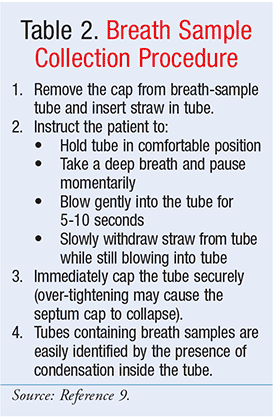
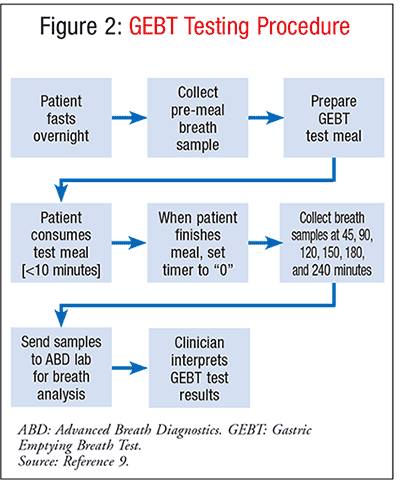
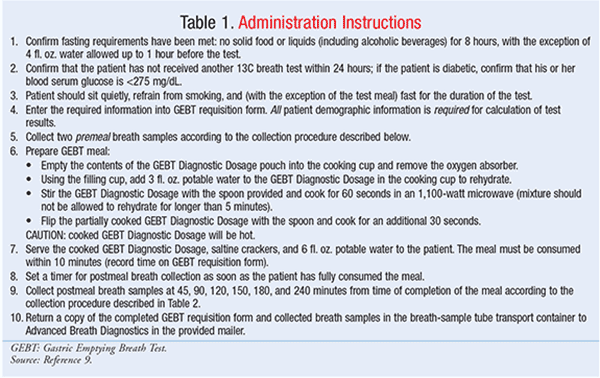
The GEBT is measured over a 4-hour time cycle. Detailed administration instructions can be found in Table 1. Multiple breath samples are obtained from the patient; sampling should be performed subsequent to an 8-hour fast. After providing multiple premeal breath samples (Table 2), the patient consumes the standard GEBT meal consisting of pasteurized scrambled egg mix containing a dose of 43 mg of 13C-Spirulina, 6 saltine crackers, and 6 ounces of potable water.5 The 13C-Spirulina is eventually passed through the GI tract to the intestines; it is in the intestines that the Spirulina is absorbed and metabolized to the 13CO2 expired in the breath. Single postmeal breath samples are subsequently collected (Table 2) at 45, 90, 120, 150, 180, and 240 minutes from the end of test-meal consumption. The post-meal breath samples are transmitted to a laboratory for assessment of 13CO2/12CO2 in each sample. By measuring the change in this ratio over time as compared to the pre-meal value, the rate of 13CO2 excretion can be calculated and the patient’s gastric-emptying rate determined.4,5 See FIGURE 2 for a diagram outlining the procedure.
Efficacy
Scintigraphy, a competitor to the GEBT, utilizes a radioisotope that is unsafe for patients who are having multiple tests performed, pregnant women, women who breast-feed, and children. Scintigraphy also requires special equipment and a specific testing site, whereas the GEBT can be implemented outside the clinical setting and breath samples are subsequently sent to a laboratory for analysis.4-6
In a clinical trial comparing the GEBT and scintigraphy, the GEBT was shown to be comparable to scintigraphy. Researchers compared the results from both the GEBT and scintigraphy and found that GEBT results agreed with scintigraphy results 73% to 97% of the time when measured from various time points during the test.4,5 The GEBT was also found to be safer than scintigraphy.
Adverse Effects/Contraindications
In the comparative study mentioned above, 13 participants complained of adverse effects.5,7 Adverse events experienced were often considered mild; these events included nausea, heartburn, diarrhea, dry heaves, abdominal pain, acid reflux, dizziness, and head cold. It was also determined later that many of these events were not related to the device. Contraindications to use of the GEBT include egg, milk, or wheat allergy, as the food product ingested contains these ingredients.5,8 Additionally, as the digestion of the product involves other organs such as the pancreas and liver, the GEBT is not recommended in patients with malabsorption, or who have diseases such as pancreatitis or hepatitis.
Conclusion
The GEBT is unique in that it provides a nonradioactive, noninvasive, orally administered product that performs as well as scintigraphy. The ease of use outside of the clinical setting also makes this device an attractive alternative to other available tests. For more information, contact Advanced Breath Diagnostics at (615) 376-5464.
REFERENCES
1. National Institutes of Health. National Institute of Diabetes and Digestive and Kidney Diseases. Gastroparesis. June 2012. www.niddk.nih.gov/health-information/health-topics/digestive-diseases/gastroparesis/Pages/facts.aspx. Accessed July 10, 2015.
2. Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398-2404.
3. Bytzer P, Talley NJ, Leemon M, et al. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989-1996.
4. Szarka L, Camilleri M, Vella A, et al. A stable isotope breath test with a standard meal for abnormal gastric emptying of solids in the clinic and in research. Clin Gastroenterol Hepatol. 2008;6:635-643.
5. FDA. Gastric emptying breath test. www.accessdata.fda.gov/cdrh_docs/pdf11/P110015b.pdf. Accessed July 21, 2015.
6. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1589-1591.
7. Abell TL, Bernstein RK, Cutts T, et al. The American Motility Society Task Force on Gastroparesis. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;4:263-283.
8. Park M, Camilleri M. Gastroparesis: clinical update. Am J Gastroenterol. 2006;101:1129-1139.
9. Gastric Emptying Breath Test [package insert]. Brentwod, TN: Advanced Breath Diagnostics, LLC; 2015.
To comment on this article, contact rdavidson@uspharmacist.com.