US Pharm. 2016;41(5)(Specialty&Oncology suppl):13-19.
ABSTRACT: Hepatitis C virus (HCV) affects more than 3% of the world population; of this number, about 160 million people have chronic HCV. Left untreated, chronic HCV slowly progresses to advanced liver disease and death from complications. New HCV treatments have improved cure rates significantly; however, many barriers to efficacy—including adverse drug reactions (ADRs), drug interactions, cost, and patient adherence—still exist. To achieve an optimal treatment result, specialty pharmacists must collaborate with physicians, insurance companies, pharmaceutical manufacturers, and the patient to select the best HCV regimen at the lowest cost; they also provide support to patients to help them adhere to treatment for the required duration. Specialty pharmacists, therefore, contribute significantly to helping HCV patients achieve an effective treatment at the lowest possible cost and with the fewest ADRs and drug interactions.
Hepatitis C virus (HCV) affects more than 3% of the global population, and approximately 160 million people have chronic HCV. In the United States, more than 3 million people have chronic HCV; about 17,000 new cases of HCV are diagnosed annually, and the virus accounts for 12,000 deaths each year.1-4 HCV, which is curable, is caused by a positive single-stranded RNA virus with six major HCV genotypes (GTs) and more than 50 minor subtypes. GT1, the most common HCV GT in the U.S., is associated with a lower rate of treatment response.1-6
Routes of HCV transmission include blood transfusion (before 1990), needle sharing (60% of new infections), sexual intercourse, mother-to-newborn transfer, and occupational exposure. Up to 85% of acute HCV cases become chronic over time.4 Acute HCV and chronic HCV are sometimes asymptomatic, but acute HCV can cause mild symptoms (malaise, nausea, jaundice, right-upper-quadrant pain) for 2 to 12 weeks, and chronic HCV can cause mild fatigue, anorexia, and nausea. Left untreated, chronic HCV slowly progresses to advanced liver disease (cirrhosis, decompensated cirrhosis, or hepatocellular carcinoma [HCC]); up to 25% of chronic HCV patients develop cirrhosis within 20 years, and each year 1% to 5% of cirrhosis patients develop HCC.1-5 The prevalence of advanced liver disease was projected to increase to 303,000 patients in 2015, and complications of cirrhosis and HCC result in annual death rates of about 4% and 1% to 5%, respectively.1-5
Choosing an Optimal HCV Treatment Regimen
HCV treatment has progressed from standard dual therapy (pegylated interferon [PEG-IFN] + ribavirin [RBV], used since 2001) to triple therapy (PEG-IFN + RBV + protease inhibitor [PI; telaprevir or boceprevir], used since 2011).3 The efficacy rate of HCV treatment is 40% to 50% for dual therapy and 50% to 75% for triple therapy. The use of oral regimens combining direct-acting antivirals from different classes (nonstructural protein 5B [NS5B] nucleotide inhibitors, NS5B nonnucleoside inhibitors, NS5A replication complex inhibitors, and NS3/4A PIs) led to an increased efficacy rate of 85% to 93% in 2013.3,7 Improvements in HCV treatment include shorter duration of therapy and fewer side effects.3,6,7 However, many factors are barriers to triple therapy, including regimen complexity, side effects, interactions, adherence, and cost.3,8 HCV management therefore requires the oversight of a healthcare team that includes a specialty pharmacist, who plays a vital role in enhancing treatment outcomes.3,9 Treatment efficacy relies on a collaboration between the prescriber, health insurer, pharmaceutical manufacturer, specialty pharmacist, and patient.3
To determine the optimal HCV treatment, the specialty pharmacist first reviews the patient’s medical history, including current diseases and medications, laboratory test results, HCV GT and subtype, baseline viral load, stage of liver disease, prior treatment and response, and history of transplant.3 The specialty pharmacist then works with the prescriber to choose the optimal cost-effective treatment regimen for the patient. Selection of an HCV regimen should be based on the patient’s GT and subtype, viral load, treatment history, and response. Step therapy should be used to keep costs as low as possible.3,4 The initial HCV treatment may be selected from the currently available regimens approved by the FDA. TABLE 1 provides the recommended HCV treatment regimens for GT1 through GT6. TABLE 2 summarizes the efficacy and duration of GT1 regimens listed in TABLE 1.
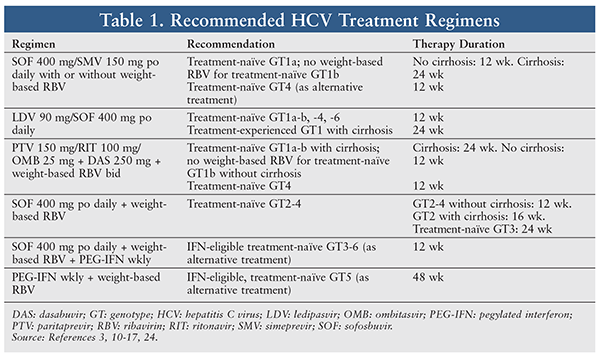
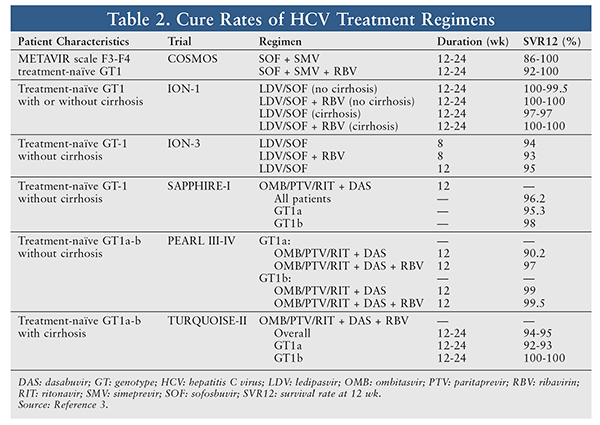
Simeprevir (SMV) 150 mg/Sofosbuvir (SOF) 400 mg: This fixed-dose regimen was approved on November 6, 2014, for treatment of chronic HCV GT1 in treatment-naïve or prior-relapse patients.8 SMV, an HCV NS3/4A PI essential for viral replication, has antiviral activity against HCV GT1, -2, -4, -5, and -6.8,10 Therefore, this regimen is also used as an alternative for GT4 patients (duration of 12 weeks). The tablet is administered once daily for 12 weeks in noncirrhotic patients and for 24 weeks in cirrhotic patients. In the COSMOS study, the SMV/SOF + RBV regimen yielded a 12-week survival rate (SVR) of 92% versus 86% for the SMV/SOF regimen. Although adding RBV to the SMV/SOF regimen improved the cure rate at 12 weeks, there was no added benefit at 24 weeks, as both regimens achieved a 100% cure rate at 24 weeks.4,10,11
Ledipasvir (LDV) 90 mg/SOF 400 mg: This regimen is used in HCV treatment–naïve GT1, -4, and -6 patients with or without cirrhosis or treatment-experienced patients without cirrhosis at a dosage of 1 tablet daily for 12 weeks. (A treatment-experienced patient is one who has failed a regimen of PEG-IFN-alfa + RBV or a regimen of an HCV PI + PEG-IFN-alfa + RBV.) An 8-week therapy duration may be considered in treatment-naïve patients without cirrhosis who have HCV RNA <6 million IU/mL. For treatment-experienced GT1 patients with cirrhosis, dosing is 1 tablet daily for 24 weeks.4 In the ION-1 study, the LDV/SOF regimen with or without RBV had a 100% cure rate at 12 weeks in GT1 treatment-naïve patients. In a phase III, open-label study of treatment-naïve HCV GT1 patients, a once-daily LDV/SOF tablet resulted in a survival rate of 97% for 12 weeks and 98% for 24 weeks.12 The addition of RBV to this regimen yielded cure rates of 97% and 99% at 12 weeks and 24 weeks, respectively; without RBV, however, the LDV/SOF regimen achieved cure rates of only 97% at 12 and 24 weeks in cirrhotic patients.12 Apparently, adding RBV increases the cure rate in cirrhotic HCV patients.10 In the ION-3 study, the LDV/SOF regimen yielded a similar cure rate at 8 weeks and 12 weeks (94% vs. 95%), but adding RBV did not increase the cure rate at 8 weeks (93% with RBV vs. 94% without RBV).13 However, in GT1 treatment-naïve noncirrhotic patients with a viral load 6 million IU/mL, the relapse rate at 8 weeks is 10 times that at 12 weeks; therefore, the 8-week LDV/SOF regimen is used only in GT1 treatment-naïve noncirrhotic patients with a viral load <6 million IU/mL.14
Ombitasvir (OMB)/Paritaprevir (PTV)/Ritonavir (RIT) + Dasabuvir (DAS) With or Without RBV: This oral regimen is used to treat HCV GT1 and -4. The dosage is 2 fixed-dose tablets of OMB 12.5 mg/PTV 75 mg/RIT 50 mg every morning + 1 tablet of DAS 250 mg + weight-based RBV twice daily. For the RBV, if the patient weighs <75 kg, the dosage is 1,000 mg; if the patient weighs 75 kg, the dosage is 1,200 mg. In the SAPPHIRE-I study, OMB/PTV/RIT/DAS + RBV administered to treatment-naïve noncirrhotic GT1 patients resulted in a 12-week cure rate of 96.2% for all patients, 95.3% for GT1a patients, and 98% for GT1b patients.4,15 In another trial (Pearl-IV), OMB/PTV/RIT/DAS + RBV given to GT1a patients had a better 12-week cure rate (97% vs 90.2% without RBV), and the rate of virologic failure was higher in the RBV-free group than in the RBV group (7.8% vs. 2.0%).16 For GT1b patients, the addition of RBV did not yield a higher cure rate (99.5% vs. 99%).16 In the TURQUOISE-II study, OMB/PTV/RIT/DAS + RBV in GT1 treatment-naïve cirrhotic patients showed similar cure rates for 12 weeks versus 24 weeks (91.8% vs. 95.9%).17 GT1a patients with a prior null response had a cure rate of 80% for 12 weeks compared with 92.9% for 24 weeks. However, GT1b patients with a prior null response had the same cure rate (100%) for either treatment duration.17 Therefore, GT1a and cirrhotic GT1b patients—but not noncirrhotic GT1b patients—require the addition of RBV.
Other Regimens: For other genotypes, SOF 400 mg daily + weight-based RBV have been used for treatment-naïve GT2–GT4 patients at a duration of 12 weeks for noncirrhotic GT2 and GT4 patients, 16 weeks for cirrhotic GT2 patients, and 24 weeks for treatment-naïve GT3 patients. A phase II trial revealed that this regimen had a 12-week cure rate of 100% for treatment-naïve and 68% for treatment-experienced GT2 and GT3 patients.18
SOF 400 mg daily + weight-based RBV + weekly PEG-IFN for 12 weeks is an alternative regimen for IFN-eligible, treatment-naïve GT3–GT6 patients. In a single-group study of SOF + PEG-IFN + RBV, patients with predominantly GT1 or GT4 HCV had an SVR of 90% at 12 weeks and an SVR of 97% for GT4–GT6 patients.19
Finally, weekly PEG-IFN + weight-based RBV is an alternative regimen for IFN-eligible, treatment-naïve GT5 patients. In various small studies, the PEG-IFN + RBV regimen shows that, at 48 weeks, GT4–GT6 patients had SVRs of 40% to 70%, 60% to 70%, and 70% to 80%, respectively; however, at 24 weeks, GT6 patients had a cure rate of just 70%.20
Monitoring Adverse Effects and Adherence
Treatment of HCV with weekly IFN and RBV for up to 1 year has low efficacy and results in many serious adverse drug reactions (ADRs)—e.g., depression, nausea, severe RBC reductions, flulike symptoms—that cause many patients to discontinue treatment before reaching the required duration. The newer HCV medications cause fewer ADRs and have greater efficacy; however, patients still find it difficult to cope with the unexpected side effects and daily disruptions that significantly impact their quality of life.21 Consultations with healthcare providers, mainly specialty pharmacists, can help patients overcome ADRs and adhere to the treatment regimen.
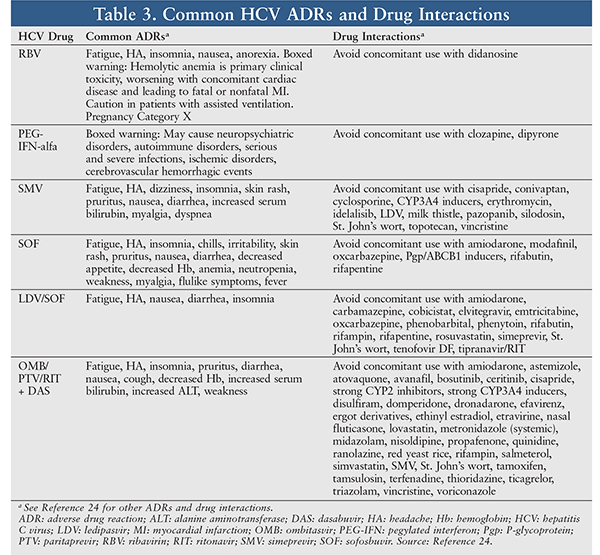
TABLE 3 lists common ADRs of typical HCV drugs and drug interactions. The specialty pharmacist should review the patient’s current medications in order to prevent serious drug interactions that would reduce the HCV drugs’ efficacy or increase their toxicity. Any concomitant medication that reduces HCV treatment effectiveness or increases toxicity should be replaced with an alternative. ADRs of the chosen HCV regimen should be reviewed and discussed with the patient. Finally, when the patient starts HCV treatment, the specialty pharmacist should monitor and respond quickly to any serious ADRs and help the patient cope with difficult situations and adhere to treatment.3
Cost Considerations and Access to Medications
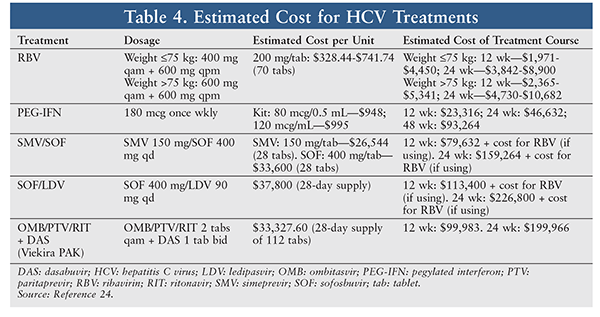
Specialty medication is costly (TABLE 4), and many patients cannot afford the medications necessary for their HCV treatment.22 However, some pharmaceutical companies have programs that allow eligible patients to obtain these medications if they cannot afford them or have no health insurance. Through Gilead’s Support Path Program, the majority of commercially insured patients can obtain Harvoni and Sovaldi for a $5 monthly copayment. The Support Path Program also helps patients locate financial assistance available from nonprofit organizations. For eligible patients without other insurance options, the Support Path Program provides Harvoni and Sovaldi at no charge.2
The Specialty Pharmacist’s Role
HCV disease management is complex and costly, with many barriers to treatment efficacy. The management team includes a specialty pharmacist and other healthcare professionals in collaboration with the patient, insurance company, and pharmaceutical manufacturer.22,23 Specialty pharmacists review patients’ clinical history and select the optimal treatment regimen; they also counsel patients about pathology, treatment regimens, ADRs, and costs and help eligible patients obtain HCV medication through pharmaceutical company–based support programs. Specialty pharmacists also help patients cope with ADRs’ effects on quality of life so that they can adhere to treatment for the necessary duration. Specialty pharmacists play a vital role in helping HCV patients achieve an effective treatment at the lowest possible cost and with as few ADRs as possible.
REFERENCES
1. Xiong W. Chronic hepatitis C pathology. Medscape. http://emedicine.medscape.com/article/1610728-overview. Assessed May 19, 2015.
2. Gilead Sciences Policy Position. Innovating and expanding access to hepatitis C treatments. www.gilead.com/~/media/files/pdfs/policy-perspectives/expanding%20access%20to%20hcv%20treatments%20102814.pdf?la=en. Accessed May 25, 2015.
3. Belperio PS, Fenrick B, Smith JP. Managed care perspectives in hepatitis C: ensuring the right patient for the right therapy. www.powerpak.com/course/preamble/111793. Accessed March 29, 2016.
4. Crowe M. The future of hepatitis C virus treatment: implications for specialty and community pharmacy. www.pharmacytimes.org/landing/595. Accessed September 1, 2015.
5. Zalesak M, Francis K, Gedeon A, et al. Current and future disease progression of the chronic HCV population in the United States. PLoS One. 2013;8:e63959.
6. Asselah T, Marcellin P. Optimal IFN-free therapy in treatment-naïve patients with HCV genotype 1 infection. Liver Int. 2015;35(suppl 1):56-64.
7. Petta S, Craxi A. Current and future HCV therapy: do we still need other anti-HCV drugs? Liver Int. 2015;35(suppl 1):4-10.
8. Infectious Diseases Society of America. Initial treatment of HCV infection. http://www.hcvguidelines.org/full-report/initial-treatment-hcv-infection. Accessed March 29, 2016.
9. Young B, Gardenier D, Martin MT, et al. Hepatitis C management: it takes a team. Medscape. www.medscape.com/viewarticle/823386. Accessed May 5, 2015.
10. Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naïve patients: the COSMOS randomised study. Lancet. 2014;384:1756-1765.
11. del Barrio Gascón C, Buti M. The potential role of simeprevir for the treatment of hepatitis C. Fut Virology. 2015;10:67-75.
12. Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014:370:1889-1898.
13. Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879-1888.
14. Harvoni (ledipasvir and sofosbuvir) product information. Foster City, CA: Gilead Sciences, Inc; February 2016.
15. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594-1603.
16. Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:183-192.
17. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973-1982.
18. Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867-1873.
19. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887.
20. Wantuck JM, Ahmed A, Nguyen MH. The epidemiology and therapy of chronic hepatitis C genotypes 4, 5 and 6. Aliment Pharmacol Ther. 2014;39:137-147.
21. Rasi M, Künzler-Heule P, Schmid P, et al. “Fighting an uphill battle”: experience with the HCV triple therapy: a qualitative thematic analysis. BMC Infect Dis. 2014;14:507.
22. Ditah I, Al Bawardy B, Gonzalez HC, et al. Lack of health insurance limits the benefits of hepatitis C virus screening: insights from the National Health and Nutrition Examination Hepatitis C follow-up study. Am J Gastroenterol. 2015;110:1126-1133.
23. Crespo J, Cabezas J, Sacristán B, et al. Barriers to HCV treatment in the era of triple therapy: a prospective multi-centred study in clinical practice. Liver Int. 2015;35:401-408.
24. Lexi-Comp Online [online database]. Hudson, OH: Lexi-Comp, Inc; 2015.
To comment on this article, contact rdavidson@uspharmacist.com.





