US Pharm. 2011;36(7):37-41.
In the United States, it is well recognized that cigarette smoking is a major cause of preventable morbidity and mortality, with numerous pharmacologic and nonpharmacologic therapies available.1 Smoking is the primary causal risk factor for at least 30% of all cancer deaths and for about 80% of deaths from pulmonary disease and for early cardiovascular disease.1 According to the 2010 Surgeon General’s Report, smoking is a major contributor to many diseases and adverse health effects, including cancer, cerebrovascular disease, coronary heart disease, atherosclerosis, respiratory diseases, pregnancy complications, low bone density, and peptic ulcer disease.2 Additionally, cigarette smoking is a primary risk factor for the development of periodontal (gum) disease; it also interferes with wound healing after periodontal surgery and dental implant placement.3,4
For years, smoking has been a major economic and public health burden. Smoking intervention is important in reducing morbidity and mortality, and it is cost-effective.5 There are various methods for smoking cessation, including behavior modification (e.g., counseling, hypnosis), alternative therapies (e.g., acupuncture), nicotine replacement products (e.g., nicotine spray, gum, inhaler, lozenge, and patch), medications (e.g., bupropion, varenicline), and the latest intervention—battery-operated electronic cigarettes (e-cigarettes). E-cigars and pipes are also available.
The World Health Organization (WHO) has termed the e-cigarette devices as electronic nicotine delivery systems (ENDS).6,7 The e-cigarette was first patented by a pharmacist in China in 2004 and since then has been available in the U.S. and other countries.8 It is estimated that there are several million users worldwide.9 Over the past 4 years, e-cigarettes have gained in popularity online and have been promoted to be a safer, savvier, and cheaper mode of smoking that delivers volatilized chemical substances such as nicotine that can be used anywhere, even in smoke-free public places.10 It has also been promoted to reduce second-hand smoke or to stop or prevent smoking by delivering nicotine and providing craving relief.11,12
Description and Mechanism of E-Cigarettes
E-cigarettes can be purchased on the Internet or through retail venues. There are two model types—manual and automatic. Different brands are available that have nonstandardized concentrations of nicotine. Most e-cigarette products come with four parts: 1) the mouthpiece or cartridge; 2) the atomizer chamber and heating coil; 3) the battery; and 4) the LED light cover (FIGURE 1). The cartridge, which is disposable and open at each end, contains the liquid (e-liquid) that will be vaporized. The e-liquid is made up of nicotine, propylene glycol, flavorings, and other medications (e.g., aminotadalafil, rimonabant).13 The liquid is vaporized by heating, which is inhaled. Below the cartridge is a lithium ion battery and a smart-chip controller with an operating mode sensor and an LED “ash” that lights up with a puff.8 Every e-cigarette includes a rechargeable battery, battery charger, and a choice of flavors (e.g., tobacco, menthol, cherry, blueberry, coffee, chocolate, strawberry, cigar, and nicotine).

Different products can be purchased, including a starter kit with refillable e-cigarettes, disposable e-cigarettes, and e-liquid to refill empty cartridges rather than buying new ones. The cost of an e-cigarette starter kit varies from $65 and up, and a five-pack of cartridges is about $12 to $15 (each cartridge = 1 pack of cigarettes).14 Automatic styles only require puffing or taking a drag to activate the atomizer. The e-liquid in the filter then heats up to produce vapor or mist. Manual e-cigarettes heat up the atomizer and create vapor only when pressing the button and inhaling.15
The strength of nicotine varies from none to 4 to 24 mg (full flavor: 16-24 mg; medium: 12-16 mg; light: 4-8 mg) in each cartridge.16 The idea is to start off with a nicotine cartridge of your personal desire of nicotine strength, such as medium, and then titrate the dosage up or down. Thereafter, the strength should slowly be reduced to the non-nicotine cartridges. The higher strength concentration may burn the throat and cause dizziness.7 No lighter is required because the e-cigarette uses a rechargeable battery to simulate the fiery end that illuminates when a puff is taken.
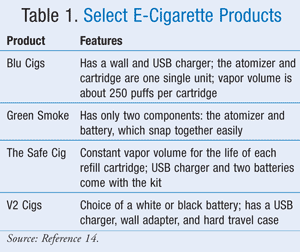
The original intent of an e-cigarette was to imitate the nicotine delivery system of a conventional cigarette without the harmful effects of tobacco smoke combustion.7 The e-cigarette looks and tastes similar to a real cigarette and gives a nicotine fix. When a button is clicked on an e-cigarette, the liquid nicotine, flavors, and chemicals present in the plastic tube heat up and an atomizer (aerosol) turns into a vapor that is puffed or inhaled and enters into the airway, which is rapidly absorbed into the systemic circulation, giving a dose of nicotine in the brain. The smoke evolving from the plastic body comes from the vaporization of propylene glycol, a humectant, which is added to the liquid. If the cartridge is refilled by the individual, the nicotine strength can be as high as 48 mg depending on where it was purchased.7 TABLE 1 lists selected e-cigarettes and their various features.14
Effects of E-Cigarettes
Questions have arisen as to what health effects e-cigarettes have on an individual. Certain clinical parameters have to be evaluated in e-cigarette users versus conventional smokers. Such parameters include the plasma nicotine levels, cardiovascular response (heart rate), periodontal disease involvement, and oral cancer incidence. It is well established that nicotine and its by-products in conventional cigarettes cause or increase the incidence of oral/head and neck cancers and periodontal diseases, and show nicotine present in the plasma.17 Additionally, e-cigarettes should not be used by pregnant women because of the nicotine. In contrast to regular cigarettes, e-cigarettes have not been found to be a risk factor for lung cancer or stroke. Although one clinical study in 2010 did not find any increase in heart rate or measurable levels of nicotine in the plasma, there are still not enough studies to show a complete profile of acute or chronic effects of e-cigarettes.18 Adverse effects of e-cigarettes can be found in TABLE 2.19

Regulation and Implications: Pros and Cons
As of January 2011, there were no federal or state regulatory polices for ENDS or e-cigarettes.20 The FDA is trying to stop the importation of e-cigarettes and wants them classified as medical devices, but a recent court ruling stated that they can only be regulated as tobacco products (the FDA is planning to appeal).20 This implies that although the FDA can review new e-cigarette products before they go on the market, there is no close monitoring because it cannot require manufacturers to conduct animal and human studies that are required for FDA approval of new drugs or medical devices.
New York State claims that manufacturers must demonstrate to the FDA that e-cigarettes are efficacious for smoking cessation.21 In response, manufacturers claim that e-cigarettes should not be part of the smoking bans in public places since there are no harmful end products, including second-hand smoke. To date, the WHO recommends that the use of ENDS in public places should be given the same restrictions as conventional smoking to reduce second-hand smoke.22
Originally, ENDS were developed with the intent of having a nicotine device similar to the conventional cigarette without the dangerous effects of tobacco smoke; however, the question arises as to whether e-cigarettes are less harmful than conventional cigarettes that have toxic chemicals in the tobacco smoke. There is concern about nicotine overdose (at doses of 60 mg) when refilling the cartridges and the development of carcinogens and even death.16,23 The FDA has found a number of toxins in cartridges, including diethylene glycol and N-nitrosamines, but at lower levels than in conventional cigarettes.15,24 Many errors and imperfections regarding the labeling and packaging of these devices were also found, including leaking of nicotine, actual amount of nicotine in the cartridge, problems with proper disposal of nicotine-containing cartridges, and mislabeling of the cartridges, wrappers, and packs.23 There are also worries about ENDS being attractive to children and adolescents because of the different flavors of liquid nicotine offered.25
Advocates of ENDS believe that they are safe because the devices deliver vaporized nicotine without the toxic combustion of tobacco and may show promise as an alternative method of smoking without the harmful effects.26 Additionally, e-cigarettes may help to suppress the craving to smoke. A clinical study found that after abstaining from smoking overnight, individuals had a decreased desire to smoke with the use of e-cigarettes (16 mg) when compared to conventional cigarettes, but there was no difference when compared to a nicotine inhaler.19
Thus far, no ENDS have been submitted to the FDA for approval of smoking cessation, so the public is unaware of the actual nicotine levels or other chemicals present. The FDA is concerned about how these devices are marketed to the public and has issued a warning about potential health risks associated with e-cigarettes, including nicotine addiction, and ingredients that may be toxic to humans.27 The FDA has also analyzed different ENDS devices and concluded that there were various levels of toxic chemicals and carcinogens, including diethylene glycol.28
To date, no clinical studies have been conducted to show either safety of e-cigarettes or their efficacy as claimed by manufacturers or the potential to cause lung cancer or stroke. There is concern whether nicotine is delivered to the lungs as claimed by the manufacturers and may actually deliver more toxic products than proposed.21 It has been questioned if there is a uniform amount of nicotine delivered to the lungs and that it might be more difficult in terms of puff strength (stronger vacuum) to produce an aerosol in e-cigarettes.29 Until more conclusive studies can be conducted, some countries, including Brazil, Canada, Uruguay, Singapore, and Turkey, have barred ENDS.6
Conclusion
E-cigarettes or ENDS are a new and novel approach for delivering nicotine without burning tobacco with the resultant harmful toxicants. The idea behind e-cigarettes is that the device delivers nicotine via a vapor without combustion or smoke. There are conflicting reports on the health effects of ENDS. More clinical studies need to be conducted before a final decision is made concerning the pharmacology, health benefits, and legal and regulatory issues.
Based on a literature review and limited clinical studies of ENDS, there seems to be a need for better regulation of these devices. It is far too easy to obtain ENDS on the Internet, not knowing exactly how they function and their harmful effects on humans. Many believe that these devices have significant concerns to the public health community. There is no regulation regarding a standardization of manufacturing of ENDS, including the design of the device and type and dosage of ingredients. Overall, more needs to be known about the possible health benefits and risks before pharmacists can recommend the use of e-cigarettes.
REFERENCES
1. Strayer SM, Rollins LK, Martindale JR. A handheld computer smoking intervention tool and its effects on physician smoking cessation counseling. J Am Board Fam Med. 2006;19:350-357.
2. 2010 Surgeon General’s Report—How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. Rockville, MD: Centers for Disease Control and Prevention; 2010. www.cdc.gov/tobacco/data_
3. Levin L, Schwartz-Arad D. The effect of cigarette smoking on dental implant and related surgery. Implant Dent. 2005;14:357-361.
4. Johnson GK, Guthmiller JM. The impact of cigarette smoking on periodontal disease and treatment. Periodontol. 2000;2007:178-194.
5. Okuyemi KS, Nollen NL, Ahluwalia JS. Interventions to facilitate smoking cessation. Am Fam Physician. 2006;74:262-271.
6. World Health Organization Study Group on Tobacco Regulation. Tobreg scientific recommendation: devices designed for the purpose of nicotine to the respiratory system in which tobacco is not necessary for their operation. In: Report on the Scientific Basis of Tobacco Regulation: Third Report of a WHO Study Group. Geneva, Switzerland: World Health Organization; 2009.
7. Kuschner WG, Reddy S, Mehrotra N, Paintal HS. Electronic cigarettes and third-hand tobacco smoke: two emerging health care challenges for the primary care provider. Int J Gen Med. 2011;4:115-120.
8. Yamin CK, Bitton A, Bates DW. E-cigarettes: a rapidly growing Internet phenomenon. Ann Intern Med. 2010;153:607-609.
9. FDA to regulate e-cigarettes as tobacco products. USA Today. April 25, 2011. http://yourlife.usatoday.com/
10. Wollscheid KA, Kremzner ME. Electronic cigarettes: safety concerns and regulatory issues. Am J Health Syst Pharm. 2009;66:1740-1742.
11. Ayers JW, Ribisl KM, Brownstein JS. Tracking the rise in popularity of electronic nicotine delivery systems (electronic cigarettes) using search query surveillance. Am J Prev Med. 2011;40:448-453.
12. Kuehn BM. FDA: electronic cigarettes may be risky. JAMA. 2009;302:937.
13. Etter JF, Bullen C, Flouris AD, et al. Electronic nicotine delivery systems: a research agenda. Tob Control. 2011;20:243-248.
14. Gray A. Best electronic cigarettes review. www.bestecigarettesreview.com. Accessed June 14, 2011.
15. Westenberger BJ. Evaluation of e-cigarettes. Center for Drug Evaluation and Research. May 4, 2009. www.fda.gov/downloads/Drugs/
16. Trtchounian A, Talbot P. Electronic nicotine delivery systems: is there a need for regulation? Tob Control. 2011;20:47-52.
17. Hyman JJ. Cigarette smoking, periodontal disease and chronic obstructive pulmonary disease. J Periodontol. 2004;75:9-15.
18. Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19:1945-1953.
19. Bullen C, McRobbie H, Thornley S, et al. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomized cross-over trial. Tob Control. 2010;19:98-103.
20. Gallegos A. FDA regulation of e-cigarettes rebuffed again. American Medical News. February 14, 2011. www.ama-assn.org/amednews/
21. Henningfield JE, Zaatari GS. Electronic nicotine delivery systems: emerging science foundation for policy. Tob Control. 2010;19:89-90.
22. WHO framework convention on tobacco control. World Health Organization. www.who.int/fctc/en/. Accessed April 16, 2011.
23. Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;4:e7524.
24. Flouris AD, Oikonomou DN. Electronic cigarettes: miracle or menace? BMJ. 2010;340:c311.
25. One on one. Smokeless cigarettes. Are “e-cigarettes” safer than regular ones? Mayo Clinic Women’s HealthSource. 2010;14(2):8. http://healthsource.
26. Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes? J Public Health Policy. 2011;32:16-31.
27. FDA warns of health risks posed by e-cigarettes. Consumer updates. FDA. July 23, 2009. www.fda.gov/ForConsumers/
28. Electronic cigarettes. Public health focus. FDA. April 25, 2011. www.fda.gov/NewsEvents/
29. Trtchounian A, Williams M, Talbot P. Conventional and electronic cigarettes (e-cigarettes) have different smoking characteristics. Tob Res. 2010;12:905-912.
To comment on this article, contact rdavidson@uspharmacist.com.






