US Pharm. 2011;36(8):HS-2-HS-8.
HACEK is an acronym for a group of organisms that are small, fastidious gram-negative bacilli.1 The HACEK organisms include Haemophilus species, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae.2,3 These organisms commonly colonize the human oropharynx as normal, indigenous flora that could cause mouth infections.4 HACEK organisms are most often associated with infective endocarditis, accounting for up to 10% of cases.1,5-7 Rarely, HACEK organisms may cause severe systemic infections.4 This article will briefly review the HACEK pathogens, resulting infections, and common treatment approaches.
Epidemiology
To grow, HACEK organisms need an atmosphere enriched with carbon dioxide.8,9 They grow well on nonspecific culture media rather than specialized agars like MacConkey’s agar, a growth medium for gram-negative bacteria.8 Typically, HACEK organisms grow slowly and are subject to extended incubation periods of 7 to 21 days.1 With the advent of improved culture media and automated culture systems, prolonged incubation may no longer be necessary; a 3- to 7-day incubation period may suffice. It is important to remember that there are other potential culture-negative species besides HACEK organisms, such as Legionella species, Bartonella species, Brucella species, and other fastidious pathogens.1,10
Haemophilus Species: Haemophilus species apart from the HACEK group include aphrophilus, haemolyticus, parahaemolyticus, parainfluenzae, paraphrophilus, and segnis. H aphrophilus, H paraphrophilus, and H segnis recently were classified, along with A actinomycetemcomitans, into the genus Aggregatibacter.11 For the purposes of this review, all of the HACEK-causing species formally in the Haemophilus genus will be referred to by the genus name. Haemophilus species have been isolated from brain abscesses, meningitis, endocarditis, otitis media, sinusitis, epiglottitis, hepatic abscesses, intra-abdominal infections, pneumonia, necrotizing fasciitis, septic arthritis, osteomyelitis, postoperative surgical infections, and urinary tract infections (UTIs).8,12
Aphrophilus and paraphrophilus species, which are short rods, are part of the normal flora found in the mouth and pharynx of humans.9 H segnis, a small pleomorphic rod that often manifests in asymmetrical filamentous forms, is part of the normal flora of the mouth as well.9 H parainfluenzae is a pleomorphic coccobacillus that accounts for 75% of the flora of the upper respiratory tract.13 H haemolyticus is often misidentified as Haemophilus influenzae, as some strains are not hemolytic, which is often the only characteristic used to differentiate the two types of bacteria.13
A Actinomycetemcomitans Bacillus: Aggregatibacter actinomycetemcomitans has been classified in various ways since its discovery.5,9 Initially known as Bacterium actinomycetem comitans, it was reclassified as Haemophilus actinomycetemcomitans, but this classification is not preferred because of the organism’s lack of similarity to H influenzae.5,9 Most recently, it has been classified in the genus Aggregatibacter while maintaining the same species name. A actinomycetemcomitans may appear singularly, in pairs, or in clumps.9 A actinomycetemcomitans grows in broth and blood agar, often in granules that are attached to the side of the dish while the center of the dish remains clear. Like other HACEK organisms, A actinomycetemcomitans is an opportunistic pathogen that has been associated with meningitis, bacteremia, septic arthritis, osteomyelitis, central nervous system abscesses, thyroid abscesses, endophthalmitis, endocarditis, pericarditis, cellulitis, parotitis, UTIs, pneumonia, and periodontal infections.5,8,9 Growth of a small number of A actinomycetemcomitans colonies may be seen at 18 to 24 hours, but at least 48 hours of growth may be required.5
C hominis Bacillus: Described as having a Pasteurella-like appearance, C hominis was first called Group II D. In later years, however, it was further characterized and renamed C hominis.14,15 C hominis is pleomorphic, appearing as pairs, chains, clusters, rosettes, or teardrops. As with other HACEK organisms, optimal growth of C hominis occurs in an environment containing supplemental carbon dioxide and high humidity.15 C hominis is part of the normal flora of the respiratory tract in about 70% of people.14,15 Additionally, C hominis may colonize the gastrointestinal tract, leading to infection.15 Nevertheless, C hominis is mainly known to cause endocarditis and meningitis.8 As opposed to those caused by Haemophilus, Aggregatibacter, and Kingella species, C hominis infections are uncommon in children.15
E corrodens Bacillus: E corrodens, originally proposed to be named Bacteroides corrodens, was first isolated from human saliva.16,17 It is part of the oral, upper respiratory, genitourinary, and gastrointestinal flora.16-19 E corrodens can cause liver, brain, intra-abdominal, and thyroid abscesses; it also can cause cellulitis, keratitis, empyema, periodontal infections, head and neck infections, UTIs, meningitis, bone and joint infections, endocarditis, and pleuropulmonary infections.8,17,18 E corrodens infections from human bites and in IV drug users have also been reported.17
K kingae Bacillus: K kingae is a member of the Neisseriaceae family.20 Formerly of the genus Moraxella, K kingae is now in the separate Kingella genus based on now-recognized differences between other members of the Moraxella genus.21 On blood agar, growth has been noted to be both convex and concave, which may be a cause of misidentification. K kingae frequently colonizes the throats of children, with the highest colonization rates occurring between 6 months and 4 years of age.22 As with the upper respiratory tract, mucus membranes also may be colonized by K kingae.23 Transmission typically occurs from person to person, and outbreaks have been reported in day care centers. Overcolonization with K kingae leads to infection, including abscesses, endocarditis, epiglottitis, bone infections, meningitis, oropharyngeal infections, and bacteremia.8,22 The majority of K kingae infections occur in children and in immunocompromised individuals. Unlike other Kingella infections, Kingella endocarditis can occur at any age, including adulthood.21,22
Treatment Approaches
The treatment of a HACEK infection is based on the location of the infection, clinical severity, and available susceptibility data. Owing to the increased frequency of beta-lactamase–producing strains of HACEK infections, these organisms should be considered resistant to ampicillin. Additionally, most HACEK infections are resistant to metronidazole, vancomycin, erythromycin, and clindamycin and generally are susceptible to third- or fourth-generation cephalosporins, trimethoprim-sulfamethoxazole, aztreonam, and fluoroquinolones.3,8 As always, local resistance patterns and isolate susceptibility should be considered before an antibiotic therapy is selected.
As previously mentioned, infective endocarditis is the most common infectious disease associated with the HACEK group. Selection of empiric treatment for culture-negative endocarditis is difficult and often involves treating for the more common bacterial causes of infective endocarditis, which may expose the patient to unnecessary drug therapy.3,24
Although there have been no large trials evaluating the best therapy for HACEK endocarditis, the American Heart Association has published recommendations (TABLE 1) for the treatment of both native and prosthetic-valve endocarditis caused by HACEK organisms.3,8 These recommendations have been endorsed by the Infectious Diseases Society of America. Ceftriaxone or ampicillin/sulbactam is the therapy of choice for patients with HACEK endocarditis. Fluoroquinolones may be considered as alternative therapy. Regardless of the agent chosen, treatment should last 4 to 6 weeks, depending upon the type of valve involved.3 Although HACEK organisms have been associated with other infections, these are rare compared with endocarditis.
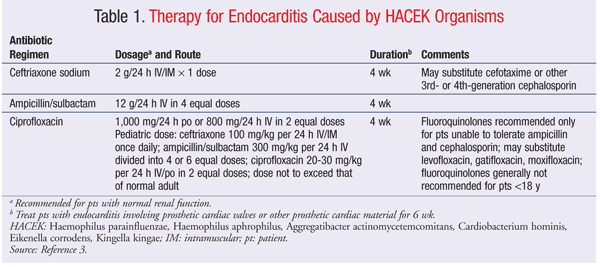
Treatment of Haemophilus Species: Most Haemophilus species are susceptible to trimethoprim-sulfamethoxazole, third-generation cephalosporins, fluoroquinolones, and aztreonam. Increased production of beta-lactamase by these organisms has been observed.12 H influenzae is commonly found in cases of otitis media, community-acquired pneumonia, and respiratory tract infections. H influenzae also may be involved in cases of bacteremia, invasive infections, and conjunctivitis. Oral antibiotics that have activity against nontypeable H influenzae (as well as Streptococcus pneumoniae and Moraxella catarrhalis) include amoxicillin/clavulanic acid, fluoroquinolones, macrolides, and various extended-spectrum cephalosporins.
H influenzae type b (Hib) can cause serious infections such as meningitis, epiglottitis, pneumonia, empyema, bacteremia, and septic arthritis. Without treatment, Hib infections may be fatal, especially in cases of meningitis and epiglottitis. Cefotaxime and ceftriaxone are the drugs most commonly used, and treatment typically lasts 7 to 10 days, although complicated cases may require 3 to 6 weeks.12,24 Conjugate vaccines for Hib have almost eradicated invasive disease in children under 5 years of age in the United States.12
Currently, four Hib-containing vaccine products are available in the U.S. Three of them consist of a polyribosylribitol phosphate conjugated to inactivated tetanus toxoid (PRP-T); the other monovalent Hib product (PedvaxHIB) is a PRP-OMP–containing vaccine. PedvaxHIB is suitable for use in all children, and it should always be used in American Indian/Alaska Native populations. All of the Hib products are interchangeable except Hiberix, which is licensed only as a booster.25 Dosing schedules for Hib immunizations differ depending upon the product used, but in general the primary series is given at 2, 4, and 6 months of age, with the final dose given any time from 12 months through 4 years.26
H parainfluenzae is associated with cases of acute bacterial exacerbation of chronic bronchitis, acute otitis media, sinusitis, and pneumonia. Other bacteria that may be involved when H parainfluenzae is present include H influenzae, M catarrhalis, and S pneumoniae. The possibility of co-infection with these organisms should be considered when antibiotic therapy is being selected. Oral agents used for infections where H parainfluenzae is suspected or confirmed include penicillins, tetracyclines, quinolones, macrolides, cephalosporins, and sulfonamides.27,28
A European study tested the antimicrobial susceptibility of H influenzae, H parainfluenzae, and M catarrhalis to 11 different antibiotics.28 Although beta-lactamase production was not studied, there was a big difference in minimum inhibitory concentration (MIC) values between amoxicillin (MIC 90 = 32 mg/L) and amoxicillin/clavulanate (MIC 90 = 2 mg/L), suggesting that beta-lactamase production is present. H parainfluenzae was 100% susceptible to cefpodoxime, cefotaxime, and levofloxacin; susceptibility also was high for amoxicillin/clavulanate, cefuroxime, and clarithromycin. Tetracyclines and sulfonamides were not studied.
Cases of endocarditis are overwhelmingly represented in literature describing infections involving H aphrophilus. Rare cases of periodontal disease, brain abscess, and vertebral osteomyelitis also have been reported. A recent case report described a patient who presented with a cerebral abscess.29 The abscess was cultured after posterior craniotomy and found to be caused by A aphrophilus. The patient was started on meropenem 2 g three times daily and metronidazole 500 mg three times daily. After 10 days, CT showed involution of the abscess cavity. After 3 weeks of meropenem plus metronidazole, the antibiotic regimen was changed to ceftriaxone 2 g daily and continued for 6 weeks. Isolates were found to be sensitive to penicillin, ampicillin, ciprofloxacin, and cephalosporins.
According to the literature, H paraphrophilus (Aggregatibacter paraphrophilus) is associated with various types of abscesses. One case report and brief review described a 3-year-old boy with an intracranial abscess caused by H paraphrophilus.30 The abscess was drained, and the patient initially was treated empirically with IV cefotaxime and metronidazole. After 2 weeks, the therapy was changed to ceftriaxone and metronidazole for 2 weeks, after which time metronidazole was discontinued and ceftriaxone was given as monotherapy for an additional 2 weeks. The total treatment duration with a third-generation cephalosporin was 6 weeks. Other cases of H paraphrophilus abscesses have been successfully treated with surgical drainage plus antibiotic therapy using combinations of cefotaxime, ciprofloxacin, meropenem, metronidazole, ceftriaxone, amoxicillin/clavulanic acid, gentamicin, flucloxacillin, piperacillin, ampicillin, and penicillin G.30-34
Treatment of A actinomycetemcomitans: A actinomycetemcomitans has been established as playing a role in periodontal disease as well as in endocarditis. The organism is commonly associated with early-onset periodontitis and has been shown to be involved in cases of chronic periodontitis.35,36 As the HACEK organism most frequently involved in infective endocarditis, A actinomycetemcomitans should be suspected in patients with endocarditis and a history of periodontal disease. A actinomycetemcomitans also has been implicated in cases of bacteremia and polymicrobial wound infections.5
The optimal treatment for A actinomycetemcomitans is unknown. The organism is usually susceptible to cephalosporins, aminoglycosides, fluoroquinolones, trimethoprim-sulfamethoxazole, and tetracycline.9,37 Resistance to ampicillin, penicillin G, vancomycin, erythromycin, and clindamycin has been noted.37 Severe A actinomycetemcomitans periodontitis is usually treated with mechanical débridement and oral tetracycline.9 A actinomycetemcomitans endocarditis has been successfully treated with amoxicillin/clavulanic acid, ampicillin, gentamicin, ceftriaxone, cefotaxime, and levofloxacin.37
Treatment of C hominis: C hominis rarely causes infections in humans.38 Only 27% of HACEK endocarditis cases are caused by C hominis.38 C hominis isolates have been reported to be susceptible to penicillin, cephalosporins, tetracycline, chloramphenicol, and aminoglycosides.38 A recent review found that 77% of patients with C hominis endocarditis have been treated successfully with penicillin alone, ceftriaxone alone, or penicillin plus an aminoglycoside for a duration of 25 to 63 days.38 It should be noted, however, that isolates with beta-lactamase production have been reported.39,40 Although C hominis is associated with endocarditis, case reports have described C hominis infections at other sites.41-43
Treatment of E corrodens: Eikenella species typically are susceptible to penicillin G, ceftriaxone, amoxicillin/clavulanic acid, trimethoprim-sulfamethoxazole, and fluoroquinolones.19,44,45 Carbapenems, tetracycline, and chloramphenicol also may be considered for treatment.45,46 Eikenella is resistant to clindamycin, cephalexin, erythromycin, and metronidazole.9,44,46 Beta-lactamase production by Eikenella is uncommon but should be considered when antimicrobial therapy is being chosen.44 Depending upon the location of the infection, surgical drainage may be more important than antibiotics alone for treatment, but co-infections with other organisms, especially streptococci, are common, and antibiotic therapy may be necessary to cover other pathogens.19,44,46
Treatment of Kingella Species: The susceptibility of Kingella species has not been well documented.22 In a retrospective review, children with proven K kingae infections were treated intravenously for 7 to 10 days, except in cases of endocarditis, which was treated for 4 to 6 weeks. Initial antibiotic regimens included ceftriaxone, amikacin/gentamicin, ampicillin, or penicillin when bacteremia was suspected; cefazolin or cefuroxime when an osteoarticular infection was suspected; and oral amoxicillin, amoxicillin/clavulanic acid, and cephalexin when occult bacteremia or pneumonia was suspected. Patients with chronic conditions were treated empirically with broad-spectrum antibiotic combinations. No treatment failures were seen with these antibiotic regimens, even in patients who initially received oral beta-lactam therapy.47
In another study, the susceptibility of 145 isolates of K kingae to eight antibiotics was tested. K kingae was susceptible to penicillins, erythromycin, gentamicin, chloramphenicol, tetracycline, and ciprofloxacin. In addition, many of the isolates were resistant to clindamycin.48
Cases of endocarditis caused by Kingella are rare and typically have a favorable outcome when treated appropriately. Successful treatment strategies for K kingae endocarditis include penicillin, ampicillin, gentamicin, tobramycin, ciprofloxacin, and ceftriaxone.
Conclusion
HACEK organisms are slow-growing, fastidious gram-negative organisms that have been associated with various infectious processes but most commonly are associated with cases of infective endocarditis. Isolates are typically susceptible to third- or fourth-generation cephalosporins, trimethoprim-sulfamethoxazole, aztreonam, and fluoroquinolones, with most HACEK organisms being resistant to metronidazole, vancomycin, erythromycin, and clindamycin.8 Beta-lactamase production has recently been observed. HACEK infections, although rare, can be extremely serious, but outcomes generally are successful if the organism is identified early and treated appropriately.
REFERENCES
1. Petti CA, Bhally HS, Weinstein MP, et al. Utility of extended blood culture incubation for isolation of Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, and Kingella organisms: a retrospective multicenter evaluation. J Clin Microbiol. 2006;44:257-259.
2. Crouch MA, Veverka A. Infective endocarditis. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 7th ed. New York, NY: McGraw-Hill Medical; 2008:1832-1833.
3. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394-e434.
4. Harrison’s Online. Chapter 140. Infections due to the HACEK group and miscellaneous gram-negative bacteria. http://accessmedicine.com/
5. Paturel L, Casalta JP, Habib G, et al. Actinobacillus actinomycetemcomitans endocarditis. Clin Microbiol Infect. 2004;10:98-118.
6. Raza SS, Sultan OW, Sohail MR. Gram-negative bacterial endocarditis in adults: state-of-the-heart. Expert Rev Anti Infect Ther. 2010;8:879-885.
7. Brouqui P, Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev. 2001;14:177-207.
8. Chu VH, Sexton DJ. HACEK. In: Schlossberg D, ed. Clinical Infectious Disease. New York, NY: Cambridge University Press; 2008:965-967.
9. Steinberg JP, Burd EM. Other gram-negative and gram-variable bacilli. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. Vol 2. 7th ed. Philadelphia, PA: Elsevier; 2010:3015-3017.
10. Baron EJ, Scott JD, Tompkins LS. Prolonged incubation and extensive subculturing do not increase recovery of clinically significant microorganisms from standard automated blood cultures. Clin Infect Dis. 2005;41:1677-1680.
11. Nørskov-Lauritsen N, Kilian M. Reclassification of Actinobacillus actinomycetemcomitans, Haemophilus aphrophilus, Haemophilus paraphrophilus and Haemophilus segnis as Aggregatibacter actinomycetemcomitans gen. nov., comb. nov., Aggregatibacter aphrophilus comb. nov. and Aggregatibacter segnis comb. nov., and emended description of Aggregatibacter aphrophilus to include V factor-dependent and V factor-independent isolates. Int J Syst Evol Microbiol. 2006;56:2135-2146.
12. Caugant DA, Selander RK, Olsen I. Differentiation between Actinobacillus (haemophilus) actinomycetemcomitans, Haemophilus aphrophilus and Haemophilus paraphrophilus by multilocus enzyme electrophoresis. J Gen Microbiol. 1990;136:2135-2141.
13. Murphy TF. Haemophilus species (including h. influenzae and chancroid). In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. Vol 2. 7th ed. Philadelphia, PA: Elsevier; 2010:2911-2918.
14. Jobanputra RS, Moysey J. Endocarditis due to Cardiobacterium hominis. J Clin Pathol. 1977;30:1033-1036.
15. Malani AN, Aronoff DM, Bradley SF, Kauffman CA. Cardiobacterium hominis endocarditis: two cases and a review of the literature. Eur J Clin Microbiol Infect Dis. 2006;25:587-595.
16. Udaka T, Hiraki N, Shiomori T, et al. Eikenella corrodens in head and neck infections. J Infect. 2007;54:343-348.
17. Ang BS, Ngan CC. Eikenella corrodens discitis after spinal surgery: case report and literature review. J Infect. 2002;45:272-274.
18. Hombach M, Frey HR, Pfyffer GE. Urinary tract infection caused by Eikenella corrodens [letter to the editor]. J Clin Microbiol. 2007;45:675.
19. Oztoprak N, Bayar U, Celebi G, et al. Eikenella corrodens, cause of a vulvar abscess in a diabetic adult. Infect Dis Obstet Gynecol. 2007;Epub:1-2.
20. Korach A, Olshtain-Pops K, Schwartz D, Moses A. Kingella kingae prosthetic valve endocarditis complicated by a paravalvular abscess. Isr Med Assoc J. 2009;11:251-253.
21. Gay RM, Lane TW, Keller DC. Septic arthritis caused by Kingella kingae. J Clin Microbiol. 1983;17:168-169.
22. Murphy TF. Moraxella catarrhalis, Kingella, and other gram-negative cocci. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. Vol 2. 7th ed. Philadelphia, PA: Elsevier; 2010:2774-2775.
23. Roiz MP, Peralta FG, Arjona R. Kingella kingae bacteremia in an immunocompetent adult host. J Clin Microbiol. 1997;35:1916.
24. Harrison’s Online. Chapter 139. Haemophilus infections. http://accessmedicine.com/
25. Briere EC, Mayer L, Messonnier N. Manual for the Surveillance of Vaccine-Preventable Diseases (5th edition, 2011). Chapter 2: Haemophilus influenzae type b (Hib). Vaccination. www.cdc.gov/vaccines/pubs/
26. Recommended immunization schedule for persons aged 0 through 6 years—United States. www.cdc.gov/vaccines/recs/
27. Chodosh S. Acute and chronic bronchitis. In: Schlossberg D, ed. Clinical Infectious Disease. New York, NY: Cambridge University Press; 2008:200-201.
28. Soriano F, Granizo JJ, Coronel P, et al. Antimicrobial susceptibility of Haemophilus influenzae, Haemophilus parainfluenzae and Moraxella catarrhalis isolated from adult patients with respiratory tract infections in four southern European countries. The ARISE project. Int J Antimicrob Agents. 2004;23:296-299.
29. Ahamed SP, Lath S, DeGabriele GJ, Mathew VT. Cerebral abscess caused by Aggregatibacter aphrophilus. Neurosciences (Riyadh). 2010;15:40-42.
30. Hoefele J, Kroener C, Berweck S, et al. Haemophilus paraphrophilus, a rare cause of intracerebral abscess in children. Eur J Pediatr. 2008;167:629-632.
31. Ariyaratnam S, Gajendragadkar PR, Dickinson RJ, et al. Liver and brain abscess caused by Aggregatibacter paraphrophilus in association with a large patent foramen ovale: a case report. J Med Case Reports. 2010;24:69.
32. Simpson AJ, Das SS, Mitchelmore IJ. Polymicrobial brain abscess involving Haemophilus paraphrophilus and Actinomyces odontolyticus. Postgrad Med J. 1996;72:297-298.
33. Haight DO, Toney JF, Greene JN, et al. Liver abscess following blunt trauma: a case report and review of the literature. South Med J. 1994;87:811-813.
34. Scerpella EG, Wu S, Oefinger PE. Case report of spinal epidural abscess caused by Haemophilus paraphrophilus. J Clin Microbiol. 1994;32:563-564.
35. Rotimi VO, Salako NO, Divia M, et al. Prevalence of periodontal bacteria in saliva of Kuwaiti children at different age groups. J Infect Public Health. 2010;3:76-82.
36. Cortelli JR, Roman-Torres CV, Aquino DR, et al. Occurrence of Aggregatibacter actinomycetemcomitans in Brazilians with chronic periodontitis. Braz Oral Res. 2010;24:217-223.
37. Wang CY, Wang HC, Li JM, et al. Invasive infections of Aggregatibacter (Actinobacillus) actinomycetemcomitans. J Microbiol Immunol Infect. 2010;43:491-497.
38. Chentanez T, Khawcharoenporn T, Chokrungvaranon N, Joyner J. Cardiobacterium hominis endocarditis presenting as acute embolic stroke: a case report and review of the literature. Heart Lung. 2011;40:262-269.
39. Lu PL, Hsueh PR, Hung CC, et al. Infective endocarditis complicated with progressive heart failure due to beta-lactamase-producing Cardiobacterium hominis. J Clin Microbiol. 2000;38:2015-2017.40. Le Quellec A, Bessis D, Perez C, Ciurana AJ. Endocarditis due to beta-lactamase-producing Cardiobacterium hominis. Clin Infect Dis. 1994;19: 994-995.
41. Kuzucu C, Yetkin G, Kocak G, Nisanoglu V. An unusual case of pericarditis caused by Cardiobacterium hominis. J Infect. 2005;50:346-347.
42. Bhan I, Chen EJ, Bazari H. Isolation of Cardiobacterium hominis from the peritoneal fluid of a patient on continuous ambulatory peritoneal dialysis. Scand J Infect Dis. 2006;38:301-303.
43. Apisarnthanarak A, Johnson RM, Braverman AC, et al. Cardiobacterium hominis bioprosthetic mitral valve endocarditis presenting as septic arthritis. Diagn Microbiol Infect Dis. 2002;42:79-81.
44. Paul K, Patel SS. Eikenella corrodens infections in children and adolescents: case reports and review of the literature. Clin Infect Dis. 2001;33:54-61.
45. Miller AT, Byrn JC, Divino CM, Weber KJ. Eikenella corrodens causing necrotizing fasciitis after an elective inguinal hernia repair in an adult: a case report and literature review. Am Surg. 2007;73:876-879.
46. Nelson MH, Aziz H. Direct inoculation osteomyelitis due to Eikenella corrodens following oral radiation therapy. Clin Lab Sci. 2007;20:24-28.
47. Dubnov-Raz G, Scheuerman O, Chodick G, et al. Invasive Kingella kingae infections in children: clinical and laboratory characteristics. Pediatrics. 2008;122:1305-1309.
48. Yagupsky P, Katz O, Peled N. Antibiotic susceptibility of Kingella kingae isolates from respiratory carriers and patients with invasive infections. J Antimicrob Chemother. 2001;47:191-193.
To comment on this article, contact rdavidson@uspharmacist.com.





