US Pharm. 2011;36(10):30-36.
Tendinitis is a familiar term used by many to refer to painful tendon lesions. Unfortunately, the word is often used incorrectly. The suffix “-itis” implies inflammation, but common causes of tendon pain rarely have an inflammatory component.1 The major cause of tendon pain has been found to be a failed healing response with tendon degeneration.2 In recent literature, the terms tendinopathy and tendinosis have been introduced to describe the pathologic changes seen in tendon injuries.3 Tendinopathy is a general term referring to any tendon injury, and tendinosis refers more specifically to injuries associated with chronic degeneration and failed healing response.1
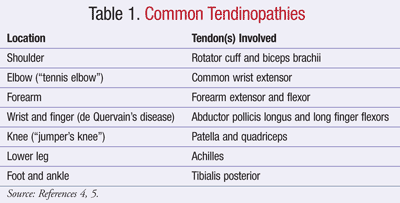
Chronic tendon injuries are most often a result of repetitive exposure to excess mechanical load.4 Eventually, the tendon is weakened and unable to adequately respond to the load. While almost any tendon can be affected, the most common areas are listed in TABLE 1. Both intrinsic and extrinsic factors contribute to the development of tendinopathy.4,5 Intrinsic factors predisposing a person to tendinopathy include increased age, male gender, and decreased range of motion or flexibility.4,5 As tendons age, they become stiffer and less capable of handling high loads, and tendon injuries may occur with only moderate exertion.4 Estrogen may play a protective role, as females have a lower incidence of tendinopathy, and postmenopausal females taking hormone replacement therapy experience less tendon damage.4 Extrinsic factors include training errors, inappropriate use of equipment, improper footwear, or inappropriate training regimens.5 Ideally, these factors should be avoided or modified to prevent the development of tendinopathy.
Pathology and Presentation
Many different models have been described to explain the pathology of an injured tendon. The most recent model suggests that there is a continuum of tendon pathology progressing through three stages from the asymptomatic tendon to tendon degeneration.1 The first stage proposed is the reactive phase, which is a short-term adaptation of an acutely overloaded tendon. This phase results in a noninflammatory thickening of the tendon.1 If the overload on the reactive tendon is not reduced appropriately, the tendon may progress to a state of disrepair, which is the second stage in the model. Tendon disrepair, or failed healing, is characterized by an increase in proteoglycan synthesis and disorganization and breakdown of the tendon matrix.1 As the tendon disorder progresses to the degenerative stage, the matrix becomes more disordered with areas of cell death and a loss of collagen. Tendons in the last stage of the continuum are past the point of spontaneous healing and are much more likely to rupture with any load applied.1
Clinical presentations vary, but most patients with tendinopathy complain of pain with activity and pain on palpation of the affected tendon.5 Since tendinopathy is often a clinical diagnosis, a thorough history regarding exercise habits and changes in work routine or recreational activities is necessary. Imaging studies are typically not indicated in the initial management of tendinopathy. Ultrasound and MRI imaging may be beneficial in confirming the diagnosis, identifying any macroscopic tears and determining the extent of involvement of surrounding structures.5 Once a diagnosis of tendinopathy has been made, treatment should be initiated and any modifiable extrinsic factors should be corrected. It is generally accepted that therapy should begin with conservative management prior to pursuing surgical interventions.
Conservative Management
Nonoperative management remains the mainstay of treatment for chronic tendinopathy.6 Although conservative treatment regimens may be unsuccessful in up to 45% of patients, it is generally accepted that patients will attempt at least 6 months of diligent physical therapy along with other conservative approaches prior to surgical intervention.6 Unfortunately, many of the conservative treatment regimens are based on anecdotal evidence, and well-designed clinical trials comparing different regimens are lacking. Nonpharmacologic and pharmacologic treatments with evidence supporting their use in tendinopathy will be discussed below.
Rest: Absolute rest achieved by immobilization is not recommended for patients with chronic tendinopathy. Tendon loading is necessary for collagen repair and tendon remodeling.5 Relative rest, which allows for maintenance of activities with reduced intensity and mechanical load, is the preferred approach. Patients should avoid activities that elicit pain from the tendon and abstain from the activity that caused the original injury during the acute phase of recovery.
Cryotherapy: Application of ice to an acute injury is a common practice. Cryotherapy provides an analgesic effect with the additional benefit of reduced metabolic rate and blood flow in the tendon, leading to decreased inflammation and acute swelling.3 The benefits of cryotherapy in chronic tendinopathies are less pronounced.
Exercises and Stretches: When discussing the role of exercise in the management of tendinopathies, it is important to first differentiate between the two most common exercise regimens, eccentric and concentric. Eccentric exercise regimens focus on activities that involve active lengthening of the muscle-tendon unit.5 Alternately, concentric exercises involve application of a load to a shortening or contracting muscle. The controlled application of strain to a damaged tendon, through eccentric exercise, appears to alter the structural and mechanical properties of the tendon, leading to increased collagen cross-linking and tendon remodeling.7 Remodeling of the tendon through regeneration is superior to tendon scarring, which is the more common outcome.7
Over 25 years ago, one of the first studies of eccentric exercise in tendinopathy reported that 87% of patients had either complete pain relief (44%) or marked improvement (43%) in pain after 6 weeks of once-daily eccentric exercises.8 While these results were significant, this study lacked a control group, and further well-designed research in this area was not pursued until recent years. These more recent randomized, controlled studies in Achilles tendinopathy have shown that the majority of patients are satisfied with their training regimen and have resumed their preinjury activity level (82%).9 Patients also reported significantly decreased pain on palpation of the tendon or with walking and experienced more asymptomatic times relative to control groups.10 While many of the clinical trials focused on Achilles tendinopathy, there have also been benefits seen in smaller trials in rotator cuff, patellar, and lateral elbow tendinopathies.11
The development of an eccentric training regimen revolves around three basic principles: length of the tendon, load applied to the tendon, and speed of tendon contraction.7 Length of the tendon can be increased by prestretching, which decreases the strain on the tendon during movement. Progressively increasing the load applied to the tendon will result in increased strength of the tendon, and increasing the speed of contraction will lead to greater force.7 While there has been little conclusive evidence on the exact design of the eccentric exercise regimen, it is apparent that patients must be highly motivated to adhere to the rigorous plan. The most common regimen used in the clinical trials includes twice-daily repetitions of exercises, 7 days a week, for 12 weeks.11 In addition, pain while exercising is not a contraindication, and patients are encouraged to continue exercising unless the pain is considered debilitating.3 Before initiating a personal exercise regimen, it is always important to consult with a primary care physician, orthopedist, or physical therapist to ensure that the intensity and frequency of the exercise is appropriate for the specific patient.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): Since the histopathology of tendinopathy has been identified as degenerative changes rather than inflammation,12 NSAID treatment has fallen out of favor. Tendinopathy-associated pain may respond to analgesics. A short trial of NSAIDs may provide additional benefit over other analgesics if an acute inflammatory component is present.13 However, long-term use of NSAIDs is not curative, and increases the risk of renal failure, cardiovascular events, gastrointestinal (GI) ulcers, and bleeding.
Corticosteroids: Corticosteroid injections are frequently used in the treatment of tendinopathies even though inflammation is not a major pathological feature. Most evidence supporting the use of steroids is anecdotal, and the limited clinical evidence suggests that local corticosteroid injections have varying effects at different tendons. The most promising evidence in favor of steroid injections for tendinopathy is for trigger finger, although studies were limited to a 1-month follow-up.14,15 For rotator cuff tendinopathy or tennis elbow, short-term pain and range-of-motion benefits have been reported, but no long-term benefit has been shown.14,16 As for Achilles tendinopathy, there is no clear benefit proven, and numerous reports of tendon rupture have been reported.17
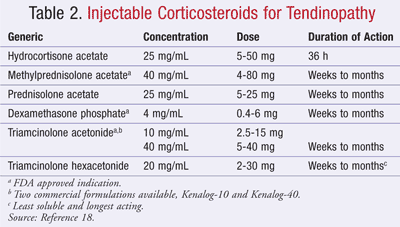
TABLE 2 lists common corticosteroids for joint and soft tissue injections. For tendon lesions, most experts recommend soluble, shorter-acting hydrocortisone preparations to decrease the long-term effects on protein synthesis and connective tissue metabolism.18 Steroid injections are often formulated with local anesthetics to decrease pain on injection.
Glyceryl Trinitrate (Nitroglycerin): Topical glyceryl trinitrate is a prodrug of nitric oxide (NO). Though the mechanism of action is not yet fully understood, animal studies show that reduced production of NO delays tendon healing.19 Theoretically, increasing exposure to NO enhances collagen synthesis by fibroblasts, resulting in stronger healing tendons.20
A recent meta-analysis found that glyceryl trinitrate is effective in decreasing pain associated with performing activities of daily living in people with chronic tendinopathies.21 When used in conjunction with eccentric exercises, decreased pain and improved range of motion have been reported.22,23 Even more impressive, 3 years after finishing a 6-month course of topical nitroglycerin therapy along with eccentric exercises, more patients with Achilles tendinopathy reported being asymptomatic than those who did eccentric exercises alone.23
Topical glyceryl trinitrate for tendinopathy is most commonly dosed at 1.25 to 5 mg/day applied to the affected tendon. The patches are changed daily, and as with all forms of nitroglycerin, the most common side effect is headache.21
Sclerosing Injections: Chronic tendinopathy is associated with areas of increased vascularity and neovascularization of affected tendons.24 These areas of hypervascularity are not associated with tendon repair but, on the contrary, have been found to be a cause of tendon pain.24 Ultrasound-guided polidocanol injections lead to sclerosis or thrombosis of targeted neovessels and surrounding nerves.25 In a double-blind randomized trial, polidocanol injections in Achilles tendinopathy have been shown to significantly reduce patient pain and increase patient satisfaction with treatment.25 Two-year follow-up of a small pilot study also reported that patients remained satisfied with results and that ultrasounds showed no new neovascularization and a more normal tendon structure.25 Very few adverse effects have been reported in patients undergoing sclerotherapy, and patients may return to a modified training regimen within 1 to 3 days of the procedure.12
While the results seem promising for sclerotherapy, most trials are small, and evidence of efficacy in different types of tendinopathies are lacking. With the currently available literature, sclerosing injections may provide the most benefit for patients with Achilles or patellar tendinopathy.25
Aprotinin: Aprotinin is an injectable, broad-spectrum, serine protease and matrix metalloproteinase (MMP) inhibitor.26 Injured tendons express higher levels of MMPs, which leads to tendon degradation and impaired healing.4,26 In patellar tendinopathy, aprotinin was found to be superior to corticosteroid and placebo injections, with a 72% positive response rate at 12 months.27 Conversely, a small underpowered study in Achilles tendinopathy showed no benefit of aprotinin injections over placebo.28 A case series analyzing 430 patients given aprotinin injections did show that 76% of patients improved, and 64% of patients found the injections to be beneficial.26 In this case series, patients with Achilles tendinopathy had higher success rates than patients with patellar tendinopathy. One concern with aprotinin is the high rate of systemic hypersensitivity reaction (2.6%) with repeat exposure; the common regimen includes 3 injections spaced 1 to 2 weeks apart.28 Data are inconsistent regarding the benefit of aprotinin in tendinopathy, and aprotinin has currently been withdrawn from the market by the manufacturer while more data are gathered on its risk in cardiac surgery, which was its only approved indication.4
Surgery
Patients experiencing persistent symptoms after 6 months of conservative therapy are considered candidates for surgical correction of tendinopathy. Surgical procedures used for tendinopathy are highly dependent on individual surgeon preference, and long-term outcomes data are lacking.29 Traditionally, surgery involves a tenotomy and excision of tendinosis tissue as well as a lengthy recovery period.30 Less invasive surgical techniques, such as “scraping,” are showing promising results with shorter recovery.30
Conclusion
Tendinopathies, or chronic tendon injuries, result from repeated exposure to mechanical stress. Early treatment of tendinopathy should consist of conservative management, including an eccentric exercise program, cryotherapy, and relative rest. Other conservative pharmacologic treatments—such as topical glycerol trinitrate, sclerosing agents, and aprotinin—show promising results. Anti-inflammatory agents are not a cornerstone of therapy, as inflammation does not play a major role in the disease process. If patients do not markedly improve after 6 months of conservative therapy, surgical correction of tendinopathies should be considered. As an easily approachable health care provider, pharmacists can provide education for patients with tendinopathies. Pharmacists should refer patients to their primary health care provider for appropriate conservative management and discourage incorrect OTC self-treatment.
REFERENCES
1. Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change confusing terminology. Arthroscopy. 1998;14:840-843.
2. Cook JL, Purdam CR. Is the tendon pathology at continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43:409-416.
3. Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology. 2006;45:508-521.
4. Rees JD, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med. 2009;37:1855-1867.
5. Almekinders LC. Tendinitis and other chronic tendinopathies. J Am Acad Orthop Surg. 1998;6:157-164.
6. Maffulli N, Longo UG, Loppini M, Denaro V. Current treatment options for tendinopathy. Expert Opin Pharmacother. 2010;11:2177-2186.
7. Maffuli N, Longo UG. How do eccentric exercises work in tendinopathy? Rheumatology. 2008;47:
1444-1445.
8. Stanish WD, Rubinovich RM, Curwin S. Eccentric exercise in chronic tendinitis. Clin Orthop Relat Res. 1986;208:65-68.
9. Mafi N, R Lorentzon R, Alfredson H. Superior short-term results with eccentric calf muscle training compared to concentric training in a randomized prospective multicenter study on patients with chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc. 2001;9:42-47.
10. Silbernagel KG, Thomee R, Thomee P, Karlsson J. Eccentric overload training for patients with chronic Achilles tendon pain—a randomised controlled student with reliability testing of the evaluation methods. Scand J Med Sci Sports. 2001;11:197-206.
11. Woodley BL, Newsham-West RJ, Baxter GD. Chronic tendinopathy: effectiveness of eccentric exercise. Br J Sports Med. 2007;41:188-199.
12. Alfredson H. The chronic painful Achilles and patellar tendon: research on basic biology and treatment. Scand J Med Sci Sports. 2005;15:252-259.
13. Paoloni JA, Milne C, Orchard J, Hamilton B. Non-steroidal anti-inflammatory drugs in sports medicine: guidelines for practical but sensible use. Br J Sports Med. 2009;43:863-865.
14. Speed CA. Fortnightly review: corticosteroid injections in tendon lesions. BMJ. 2001;323:382-386.
15. Nimigan AS, Ross DC, Gan BS. Steroid injections in the management of trigger fingers. Am J Phys Med Rehabil. 2006;85:36-43.
16. Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomized controlled trials. Lancet. 2010;376:1751-1767.
17. Blanco I, Krähenbühl S, Schlienger RG. Corticosteroid-associated tendinopathies: an analysis of the published literature and spontaneous pharmacovigilance data. Drug Safety. 2005;28:633-643.
18. Articular and periarticular corticosteroid injections. Drug Ther Bull. 1995;33:67-70.
19. Cumpston M, Johnston RV, Wengier L, Buchbinder R. Topical glyceryl trinitrate for rotator cuff disease (review). Cochrane Database Syst Rev. 2009;(3):CD006355.
20. McCallum SD, Paoloni JA, Murrell GA. Five-year prospective comparison study of topical glyceryl trinitrate treatment of chronic lateral epicondylosis at the elbow. Br J Sports Med. 2011;45:416-420.
21. Gambito ED, Gonzalez-Suarez CB, Oquiñena TI, Agbayani RB. Evidence on the effectiveness of topical nitroglycerin in the treatment of tendinopathies: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2010;91:1291-1305.
22. Paoloni JA, Appleyard RC, Nelson J, Murrell GA. Topical glyceryl trinitrate application in the treatment of chronic supraspinatus tendinopathy: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2005;33:806-813.
23. Paoloni JA, Murrell GA. Three-year followup study of topical glyceryl trinitrate treatment of chronic noninsertional Achilles tendinopathy. Foot Ankle Int. 2007;28:1064-1068.
24. Fenwick SA, Hazleman BL, Riley GP. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 2002;4:252-260.
25. Alfredson H, Ohberg L. Sclerosing injections to areas of neo-vascularization reduce pain in chronic Achilles tendinopathy: a double-blind randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2005;13:338-344.
26. Orchard J, Massey A, Brown R, Cardon-Dunbar A. Successful management of tendinopathy with injections of the MMP-inhibitor aprotinin. Clin Orthop Relat Res. 2008;466:1625-1632.
27. Capasso G, Testa V, Maffulli N, et al. Aprotinin, corticosteroids, and normosaline in the management of patellar tendinopathy in athletes: a prospective randomized study. Sports Exer Inj. 1997;3:111-115.
28. Brown R, Orchard J, Kinchington M, et al. Aprotinin in the management of Achilles tendinopathy: a randomized controlled trial. Br J Sports Med. 2006;40:275-279.
29. Tallon C, Coleman BD, Khan KM, Maffulli N. Outcome of surgery for chronic Achilles tendinopathy. A critical review. Am J Sports Med. 2001;29:315-320.
30. Alfredson H. Ultrasound and Doppler-guided mini-surgery to treat midportion Achilles tendinosis: results of a large material and a randomized study comparing two scraping techniques. Br J Sports Med. 2011;45:407-410.
To comment on this article, contact rdavidson@uspharmacist.com.






