Adverse drug reactions (ADRs) are a major health concern, and occur in 0.1% to 1% of patients taking systemic medications.1,2 The incidence of fatalities due to all drug reactions for hospitalized patients has been documented to be 0.3%.2 The skin is the largest organ in the body, and adverse skin reactions due to drug exposure are a common problem. Approximately 2% of drug-induced skin eruptions meet the World Health Organization definition of a serious reaction.1,3 The exact mechanism for many of the drug-induced cutaneous diseases is not fully understood and may result from both immune and nonimmune mechanisms. Properties of a drug that increase the risk of a drug-induced hypersensitivity reaction are: 1) molecular weight >4,000 Da (e.g., insulin, erythropoietin); 2) presence of foreign proteins or large polypeptides of nonhuman origin (streptokinase, beef or pork insulin, chimeric/murine-derived monoclonal antibodies); or 3) the ability of the parent drug or its active metabolite to bind to a carrier protein and form a complete antigen (penicillins and sulfonamides).4
Drug-induced skin disorders are often classified as either acute or chronic. Acute diseases include erythematous eruptions; urticaria, angioedema, and anaphylaxis; fixed-drug eruptions; hypersensitivity syndrome; Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN); warfarin-induced skin necrosis; vasculitis; serum sickness–like reaction; acute generalized exanthematous pustulosis (AGEP); and photosensitivity. Chronic disorders include drug-induced lupus, drug-induced acne, and pigmentary changes.
Acute Drug-Induced Skin Disorders
Erythematous Reactions: These reactions are the most common ADRs involving the skin.5 This eruption is considered a type IV delayed cell-mediated hypersensitivity reaction. The eruption typically occurs 4 to 14 days after the causative drug is initiated; however, the reaction may occur 1 to 2 days after discontinuation of the drug.1,5 Upon a second exposure, the eruption may occur more rapidly.
Lesions are symmetric erythematous macules or papules, which may be pruritic and usually develop on the trunk or upper extremities before progressing and becoming more confluent.5,6 Patients may experience a low-grade fever; however, mucous membranes are not typically involved.
Drugs that are more likely to cause this type of eruption include penicillins, cephalosporins, sulfonamides, anticonvulsants, and allopurinol (TABLE 1).5,6 Primary treatment involves discontinuing the causative agent; however, if the drug is required for essential therapy, consideration may be given to continuation of the agent unless symptoms associated with the eruption suggest a more serious reaction. Topical corticosteroids, systemic corticosteroids, or antipruritic agents may also be considered. The eruption generally resolves within 7 to 14 days after the causative agent is discontinued.6 The rash should be monitored for the first 48 hours to ensure that more severe complications/reactions are not occurring.5 Differential diagnoses for patients presenting with this eruption include viral eruptions (e.g., Epstein-Barr virus, herpesvirus type 6), eruptions from a bacterial toxin, acute graft-versus-host disease, and Kawasaki disease.5
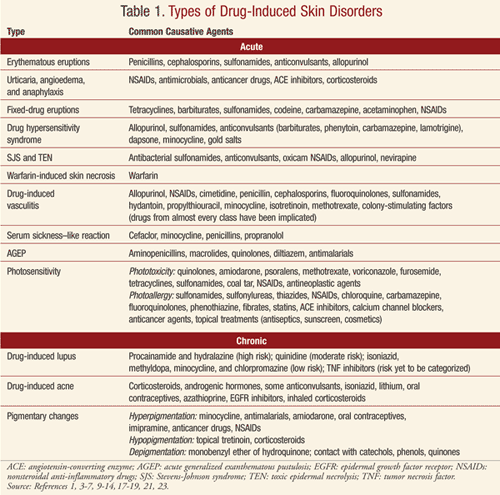
Urticaria, Angioedema, and Anaphylaxis: Urticaria (hives) is a common, acute, transient reaction sometimes referred to as the cutaneous expression of anaphylaxis.5,6 It is characterized by pruritic monomorphic erythematous and edematous papules and plaques.1,5,6 The onset of symptoms is rapid, sometimes within minutes, and the papules and plaques last from a few hours to 24 hours. New lesions, however, may continually develop. In contrast, angioedema is defined by involvement of dermal and subcutaneous tissues and is described as pale or pink swelling that affects the face, buccal mucosa, tongue, larynx, and pharynx. Anaphylaxis may complicate urticaria and angioedema and may involve additional body systems, leading to shock and death. Urticaria, angioedema, and anaphylaxis are a consequence of either an immunoglobulin E (IgE)–mediated type 1 hypersensitivity reaction or an anaphylactoid mechanism involving histamine or other inflammatory mediators. Management of this reaction consists of discontinuing the causative agent. Histamine receptor (H1) blockers, systemic corticosteroids, and epinephrine may also be required acutely.
Many drugs have been implicated in causing urticaria and/or angioedema (TABLE 1).1,4-6 Examples of anaphylactoid reactions include responses to radiocontrast media, opiate-induced urticaria, and vancomycin-induced red man syndrome.
Fixed-Drug Eruptions: These eruptions present as pruritic, red, raised lesions that may blister or develop into plaques.1,6 A burning or stinging sensation may also be noted.6 Lesions typically develop in minutes to days of drug initiation and typically resolve within days; however, hyperpigmentation may remain for months.1,5 The lesions may develop anywhere on the body and may include the mucous membranes.1 When the causative agent (TABLE 1) is readministered, the lesions recur in the same area as the primary eruption.1,6 Removal of the offending drug is the primary treatment.1 The mechanism for this eruption is unknown.1,5,6
Drug Hypersensitivity Syndrome: This syndrome, also known as drug rash with eosinophilia and systemic symptoms (DRESS), is a severe exanthematous eruption with fever, lymphadenopathy, and multi-organ involvement.5,6 DRESS occurs more frequently in persons of African descent and may be fatal if not appropriately treated. Rash and fever are usually the first symptoms. The face, upper trunk, and extremities are originally involved, with inclusion of facial edema.6 Severe hepatitis is responsible for many of the fatalities associated with this syndrome. Predominant eosinophilia is common, and aminotransferase, alkaline phosphatase, and/or bilirubin levels are elevated in approximately 50% of patients.5 DRESS typically occurs 1 to 6 weeks after introduction of the causative agent.5,6
Drugs commonly associated with DRESS are listed in TABLE 1.5,6 The incidence of the syndrome is estimated to be between 1 in 1,000 and 1 in 10,000 for patients exposed to anticonvulsants.5 Early discontinuation of the offending drug is critical but may not lead to a complete recovery. Treatment may include topical corticosteroids for skin symptoms and systemic corticosteroids when the heart and lungs are affected. A relapse of the rash and hepatitis may occur when corticosteroids are tapered. The rash and visceral involvement may remain for several weeks after removal of the offending drug.5
Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN): SJS and TEN are rare, life-threatening syndromes.5-7 The eruptions are drug-induced approximately 70% of the time.5 The incidence of SJS is estimated to be 1 to 7.1 cases per million person-years, while the incidence of TEN is thought to be 0.4 to 1.2 cases per million person-years.5,7,8 The risk is greatest in those patients infected with HIV.
In much of the literature, SJS and TEN are considered a spectrum of drug-induced skin disorders.5,7,8 The definitions of the two syndromes have evolved over the years. The percentage of skin detachment determines the classification. Less than 10% of body surface area (BSA) with skin detachment is classified as SJS, while 10% to 30% of affected BSA is referred to as SJS/TEN overlap, and greater than 30% of affected BSA is classified as TEN.
The exact mechanisms are not understood; however, early lesion immunopathologic patterns infer a cell-mediated cytotoxic reaction to epidermal cells.5 A genetic propensity for SJS and TEN may exist, as documented by a strong association between the human leukocyte antigen HLA-B*1502 allele and carbamazepine-induced SJS, and between the HLA-B*5801 allele and allopurinol-induced SJS in the Han Chinese population.5,7
Symptoms are very acute, and begin within 4 weeks of drug exposure.5,6 Symptoms have also been documented to occur days after drug withdrawal. The eruption occurs even more rapidly when the causative agent is rechallenged. Initial symptoms include a prodromal phase of fever, sore throat, and stinging eyes.5-7 The skin blisters and mucous erosions occur 1 to 2 days later, with extensive epidermal detachment and sloughing. The rash may cover the entire body. Initially, the lesions are irregularly shaped, erythematous, purpuric macules that progressively coalesce. Patients exhibit a positive Nikolsky’s sign and flaccid blisters form. Necrotic epidermis detachment occurs. The mucous membranes (buccal, ocular, nasal, and genital) are affected in at least 85% of patients.5,8 Additionally, epithelium of the gastrointestinal and respiratory tracts may be involved. Patients may also have increased hepatic enzymes and leukopenia; however, the syndromes are not typically associated with eosinophilia. A marked loss of fluids, a drop in blood pressure, electrolyte disturbances, and infection may occur.6 SJS is fatal in 5% to 10% of patients and TEN is fatal in >30% of patients.5,7 Discontinuation of the causative agent is vital. Treatment is symptomatic and supportive.5-8 No other treatments are universally accepted, as the use of corticosteroids and other therapies is controversial.5-7
Over 100 drugs have been implicated as a cause of SJS or TEN.3,5,6,9 The more commonly associated agents are provided in TABLE 1.3,5-7,9
Warfarin-Induced Skin Necrosis: Warfarin tissue necrosis is a rare but serious disorder that may occur 3 to 5 days after the initiation of warfarin.5 Red, painful plaques form that develop into necrosis, hemorrhagic blisters, and ulcers. Necrosis occurs in approximately 1 in 10,000 patients exposed to warfarin. Patients are at increased risk if a hereditary deficit in protein C is present, due to the hypercoagulable state during initiation of therapy. The mainstay of treatment is typically supportive. Warfarin should be discontinued and vitamin K, heparin, and monoclonal antibody–purified protein C concentrate administered.
Drug-Induced Vasculitis (DIV): This is defined as any case of inflammatory vasculitis caused by a specific drug.10 The exact mechanism is unknown.5,10 Patients may present with palpable purpuric lesions or a maculopapular rash. Ulcers, nodules, hemorrhagic blisters, or Raynaud’s disease may also be present, and additional organ systems may be involved.5 DIV may occur 7 to 21 days after initial drug exposure; however, the interval may be variable.5,11 Withdrawal of the causative agent (TABLE 1) may lead to rapid resolution. For more severe cases, corticosteroids or immunosuppressive drugs may be required.10 Drugs from almost every class have been associated with DIV.
Serum Sickness–Like Reactions: These reactions present with fever, an urticarial rash, arthralgias, and lymphadenopathy.5,6 The reaction occurs 1 to 3 weeks post initial drug exposure. Immune complexes, vasculitis, and renal lesions are not present. It is estimated that 1 in 2,000 children exposed to cefaclor may experience this reaction.5 Additionally, minocycline, penicillins, and propranolol have been documented to induce a serum sickness–like reaction (TABLE 1). The causative agent should be discontinued. Systemic corticosteroids may be required for treatment over 5 days for severe symptoms.4
Acute Generalized Exanthematous Pustulosis (AGEP): AGEP is a rare, acute, pustular eruption.1,5,6,12 While the etiology may be viral or a reaction to mercury, greater than 90% of cases are drug induced.1,5 AGEP is characterized by a fever and diffuse erythema. Burning and itching accompany the eruption. Patients may experience facial edema, swelling of the hands, and mucous membrane involvement. Small and mostly nonfollicular pustules develop. The eruption may last 1 to 2 weeks and is followed by superficial desquamation. Drugs frequently associated with AGEP are included in TABLE 1. Treatment consists of drug withdrawal, topical corticosteroids, and occasionally a systemic antipruritic agent or brief use of systemic corticosteroids.
Photosensitivity: This is an adverse cutaneous reaction triggered by doses of sunlight that are normally harmless. It may be idiopathic or result from either endogenous photosensitizers or exogenous causes, such as drugs.5,13-16 Photosensitivity reactions may manifest as either a photo-allergic or phototoxic reaction. Some drugs are capable of producing both types of reactions.
Phototoxic reactions are common and often predictable.5,13,16 Drugs that induce a phototoxic reaction absorb ultraviolet A (UVA) light; no immunologic mechanisms are involved.5,6,14,15 Phototoxicity is characterized by rapid onset of a burning sensation.14,15 Clinically, patients present with an exaggerated sunburn, followed by hyperpigmentation.5,13 This reaction occurs only on sun-exposed skin. Less common clinical forms of the reaction are photo-onycholysis (phototoxicity involving the nails) and pseudoporphyria (a bullous photosensitivity disorder). Photoallergy is less common than phototoxicity and is a result of cell-mediated hypersensitivity.5,6,13,14 Photoallergy occurs from UVA transformation of drugs into allergens.6 This reaction may involve exposed skin and skin that is not exposed to the sun.5,6,13,14 Unlike the more immediate phototoxic reaction, photoallergy may not present until 24 to 72 hours post sun exposure. A photoallergy clinically appears as an acute, subacute, or chronic dermatitis.13
Many drugs have been documented to cause photosensitivity reactions (TABLE 1). Treatment includes discontinuation of the causative agent and avoidance of sun exposure.5,6 Topical corticosteroids and systemic antipruritic therapies may be utilized in some circumstances. The use of sunscreens that block both UVA and UVB is important; however, in some patients sunscreen is the causative agent.
Chronic Drug-Induced Skin Disorders
Drug-Induced Lupus (DIL): This rare adverse effect occurs in approximately 15,000 to 30,000 individuals per year in the United States.17 Though several hypotheses have been postulated for the development of DIL, the pathogenic mechanism remains unclear. Drugs considered lupus inducing vary in pharmacologic and chemical characteristics, and some agents have stronger evidence for a causal relationship. Drugs associated with lupus are categorized as high risk, moderate risk, low risk, and risk yet to be categorized (TABLE 1).
The most prevalent clinical findings of DIL are musculoskeletal (e.g., arthralgias, myalgias, arthritis) and constitutional (e.g., fever, malaise, anorexia, weight loss) symptoms.17,18 Certain agents are more closely linked with specific clinical findings of hepatic abnormalities, pleuritis, and cutaneous symptoms.17 Cutaneous symptoms such as the classic butterfly rash are rare in DIL, except in the case of quinidine and hydralazine, which have incidence rates of 39% and 10% to 34%, respectively. The onset of signs and symptoms of DIL can be acute, but is more often seen as a slow, gradual process, developing months after initiation of the causative agent.17,18 Rechallenge with the offending agent may produce symptoms within 1 to 2 days.17
Subacute cutaneous lupus erythematosus (SCLE), a form of drug-induced lupus, is most commonly associated with antihypertensives (calcium channel blockers, ACE inhibitors, beta-blockers, and thiazide diuretics), HMG-CoA reductase inhibitors, oral antifungals (terbinafine, griseofulvin), antidepressants (bupropion), and tumor necrosis factor (TNF) inhibitors (etanercept, infliximab, adalimumab).5,19 Drug-induced SCLE (DI-SCLE) presents with similar clinical and serologic symptoms as idiopathic lupus20; the typical rash is photodistributed in an annular or a papulosquamous form, with or without scaling.19,21
Systemic lupus erythematosus (SLE) typically presents in women of childbearing age, whereas individuals presenting with DIL are characteristically of advanced age, with an equal distribution between males and females (with the exception of minocycline-induced lupus), and Caucasian.18,20 DIL may also have a higher predominance in individuals who are slow drug acetylators. The risk of the development of DIL does not preclude the use of associated agents in clinical decisions because of its reversibility. DIL typically resolves within weeks after the removal of the causative agent.17,18 Corticosteroids or immunosuppressive therapy may be required in more severe cases, but typically is not necessary.
Drug-Induced Acne (Acneiform Eruption): Drug-induced acne accounts for approximately 1% of drug-induced skin reactions.5 Pustular eruptions are typically observed on the face and upper trunk.5,6 Comedones are not commonly documented with this type of outbreak. The eruption occurs 1 to 3 weeks after the causative agent is initiated. Drug-induced acne is associated with the use of various agents (TABLE 1). Inhaled corticosteroids in patients with asthma have also been documented to cause acne.22 Topical acne treatment may be utilized to treat the eruption, which is typically reversible once the causative drug is withdrawn.4,22
Drug-Induced Pigmentary Changes: Pigmentary changes induced by various drugs may be caused by different mechanisms, including the enhancement of melanin production, the deposition of drugs or metabolites, or the postinflammatory changes secondary to phototoxicity.5 The effects are more likely to be observed on areas exposed to the sun. Drugs commonly associated with hyperpigmentation, hypopigmentation, and depigmentation are given in TABLE 1.5 Pigmentary changes may fade over time or may be permanent in a small number of patients.23

Conclusion
While not all skin reactions are drug induced, it is important for health care providers to recognize the characteristics and common causative agents for various acute and chronic drug-induced skin disorders. Patients presenting with a skin rash or skin lesions should be thoroughly assessed for the possibility of such a relationship and, if one is found, should be treated (if necessary) and educated regarding the drug-induced skin disorder (TABLE 2). The reaction should be properly documented, and if it is allergy mediated, the patient should be counseled to avoid further exposure. The likelihood of developing a drug-induced skin disorder should not preclude the use of particular agents in most individuals; however, extensive efforts should be taken by all members of the health care team to ensure safe use of medications in those individuals who have previously experienced a drug-induced skin disorder.
REFERENCES
1. McKenna JK, Leiferman KM. Dermatologic drug reactions. Immunol Allergy Clin North Am. 2004;24:
399-423.
2. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse
drug reactions in hospitalized patients: a meta-analysis of prospective
studies. JAMA. 1998;279:1200-1205.
3. Roujeau JC, Kelly JP, Naldi L, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333:1600-1607.
4. Sylvia LM. Drug allergy, pseudoallergy, and cutaneous diseases. In: Tisdale JE, Miller DA, eds. Drug-Induced Diseases: Prevention, Detection, and Management. 2nd ed. Bethesda, MD: American Society of Health-System Pharmacists; 2010:51-97.
5. Valeyrie-Allanore L, Sassolas B, Roujeau JC. Drug-induced skin, nail, and hair disorders. Drug Saf. 2007;30:1011-1030.
6. Law RM, Law DTS. In: Dipiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York, NY: The McGraw Hill Companies, Inc; 2008:1661-1672.
7. Chia FL, Leong KP. Severe cutaneous adverse reactions to drugs. Curr Opin Allergy Clin Immunol. 2007;7:305-309.
8. Letko E, Papaliodis DN, Papaliodis GN, et al.
Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of the
literature. Ann Allergy Asthma Immunol. 2005;94:419-436.
9. Wolf R, Orion E, Marco B, Matz H. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171-181.
10. Merkel PA. Drug-induced vasculitis. Rheum Dis Clin North Am. 2001;27:849-862.
11. ten Holder SM, Joy MS, Falk RJ. Cutaneous and systemic manifestations of drug-induced vasculitis. Ann Pharmacother. 2002;36:130-147.
12. Mockenhaupt M. Severe drug-induced skin reactions: clinical pattern, diagnostics and therapy. J Dtsch Dermatol Ges. 2009;7:142-162.
13. Gould JW, Mercurio MG, Elmets CA. Cutaneous photosensitivity diseases induced by exogenous agents.
J Am Acad Dermatol. 1995;33:551-573.
14. Dufner KS, Buss LA, Kizito J. Drug-induced photosensitivity. Hosp Pharm. 2006;41:196-206.
15. Vassileva SG, Mateev G, Parish LC. Antimicrobial photosensitive reactions. Arch Intern Med. 1998;158:1993-2000.
16. Allen JE. Drug-induced photosensitivity. Clin Pharm. 1993;12:580-587.
17. Borchers AT, Keen CL, Gershwin ME. Drug-induced lupus. Ann N Y Acad Sci. 2007;1108:166-182.
18. Rubin RL. Drug-induced lupus. Toxicology. 2005;209:135-147.
19. Chang C, Gershwin ME. Drug-induced lupus erythematosus. Drug Saf. 2011;34:357-374.
20. Vasoo S. Lupus. Lupus. 2006;15:757-761.
21. Hsu S, Le EH, Khoshevis MR. Differential diagnosis of annular lesions. Am Fam Physician. 2001;64:289-296.
22. Monk B, Cunliffe WJ, Layton AM, Rhodes DJ. Acne induced by inhaled corticosteroids. Clin Exp Dermatol. 1993;18:148-150.
23. Dereure O. Drug-induced skin pigmentation: epidemiology, diagnosis and treatment. Am J Clin Dermatol. 2001;2:253-262.
To comment on this article, contact rdavidson@uspharmacist.com.





