US Pharm. 2012;37(6):26-29.
Chronic kidney disease (CKD) is defined as persistent kidney damage accompanied by a reduction in the glomerular filtration rate (GFR) and the presence of albuminuria. The prevalence of CKD has steadily increased over the past two decades, and was reported to affect over 13% of the U.S. population in 2004.1 In 2009, more than 570,000 people in the United States were classified as having end-stage renal disease (ESRD), including nearly 400,000 dialysis patients and over 17,000 transplant recipients.2 A patient is determined to have ESRD when he or she requires replacement therapy, including dialysis or kidney transplantation. The rise in incidence of CKD is attributed to an aging populace and increases in hypertension (HTN), diabetes, and obesity within the U.S. population. CKD is associated with a host of complications including electrolyte imbalances, mineral and bone disorders, anemia, dyslipidemia, and HTN. It is well known that CKD is a risk factor for cardiovascular disease (CVD), and that a reduced GFR and albuminuria are independently associated with an increase in cardiovascular and all-cause mortality.3,4
HTN has been reported to occur in 85% to 95% of patients with CKD (stages 3-5).5 The relationship between HTN and CKD is cyclic in nature. Uncontrolled HTN is a risk factor for developing CKD, is associated with a more rapid progression of CKD, and is the second leading cause of ESRD in the U.S.6,7 Meanwhile, progressive renal disease can exacerbate uncontrolled HTN due to volume expansion and increased systemic vascular resistance. Multiple guidelines discuss the importance of lowering blood pressure (BP) to slow the progression of renal disease and reduce cardiovascular morbidity and mortality.8-10 However, in order to achieve and maintain adequate BP control, most patients with CKD require combinations of antihypertensive agents; often up to three or four medication classes may need to be employed.11
Pathophysiology
Hypertension is one of the leading causes of CKD due to the deleterious effects that increased BP has on kidney vasculature. Long-term, uncontrolled, high BP leads to high intraglomerular pressure, impairing glomerular filtration.12,13 Damage to the glomeruli lead to an increase in protein filtration, resulting in abnormally increased amounts of protein in the urine (microalbuminuria or proteinuria).12,13 Microalbuminuria is the presentation of small amounts of albumin in the urine and is often the first sign of CKD. Proteinuria (protein-to-creatinine ratio ≥200 mg/g) develops as CKD progresses, and is associated with a poor prognosis for both kidney disease and CVD.3,4,14
As discussed previously, the relationship between CKD and HTN is cyclic, as CKD can contribute to or cause HTN. Elevated BP leads to damage of blood vessels within the kidney, as well as throughout the body. This damage impairs the kidney’s ability to filter fluid and waste from the blood, leading to an increase of fluid volume in the blood—thus causing an increase in BP.
Staging of CKD
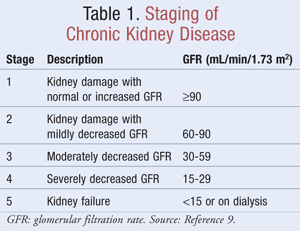
The estimated GFR, which helps clinicians determine how well the kidneys are filtering waste, is used in the staging of CKD. The National Kidney Foundation defines CKD as either kidney damage, identified by markers in the urine or blood or by imaging, with or without changes in the GFR, or a GFR <60 mL/min/1.73 m2 for a minimum of 3 months.9 TABLE 1 depicts the staging criteria as determined by the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines.9
Goals of Therapy
Patients with nondiabetic and diabetic CKD should have a target BP goal of <130/80 mmHg.8-10 Ultimately, the rationale for lowering BP in all patients with CKD is to reduce both renal and cardiovascular morbidity and mortality. Maintaining BP control and minimizing proteinuria in patients with CKD and HTN is essential for the prevention of the progression of kidney disease and the development or worsening of CVD.8,9
Recent literature suggests that BP targets in diabetic and nondiabetic CKD may need to be individualized based on the presence of proteinuria. Some trials have failed to show a reduction in cardiovascular or renal outcomes in diabetic and nondiabetic patients with CKD when a BP target of <130/80 mmHg is achieved compared to lowering BP to <140/90 mmHg.15,16 However, patients who have proteinuria are less likely to experience a decline in renal function, kidney failure, or death when the lower BP target is achieved.15,17 It is likely that future guidelines may include a lower BP goal, <130/80 mmHg, for patients with proteinuria, but maintain a goal of <140/90 mmHg for patients without proteinuria.
Treatment
Agents that not only lower BP but also reduce proteinuria are recommended as first-line therapy for most patients with CKD and HTN; data indicate there may be significant long-term benefits in both cardiovascular and renal outcomes when proteinuria is decreased.9 Several classes of antihypertensive agents may have a role in the treatment of CKD and HTN. Agents that target the renin-angiotensin-aldosterone system (RAAS), such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), are generally considered first-line antihypertensive therapy for this patient population.8,9,18 TABLE 2 provides guidance on recommended antihypertensive agents for patients with CKD with or without diabetes and with or without proteinuria.
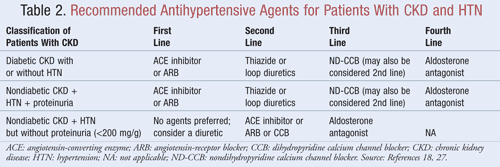
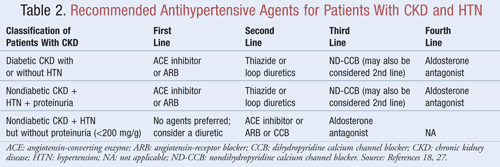
ACE Inhibitors or ARBs: Studies have shown that antihypertensive agents that target the renin-angiotensin system prevent kidney decline more so than other agents, even when achieving similar BP goals.19 These results were found primarily in patients with proteinuria, whereas the benefit was less substantial for those without proteinuria. Based on these findings, guidelines recommend ACE inhibitor or ARB therapy as first-line treatment for those with diabetes or those presenting with nondiabetic kidney disease, HTN, and proteinuria. Data indicate that ACE inhibitors and ARBs are equally effective in lowering BP and reducing proteinuria.20 A recent meta-analysis suggests that ACE inhibitor therapy may provide superior benefit over ARB therapy for the treatment of HTN due to a 10% reduction in all-cause mortality.21 These results were determined for patients with HTN and did not apply to patients with additional comorbidities such as CKD. Therefore, selection of one agent over another will depend on patient-specific factors such as potential for side effects and cost. Treatment with both an ACE inhibitor and an ARB is not recommended, as this combination has been shown to worsen kidney function. Combination ACE inhibitor and ARB therapy did not reduce cardiovascular mortality or morbidity in comparison to monotherapy of an ACE inhibitor.20,21
ACE inhibitors and ARBs are generally well tolerated. ACE inhibitors may cause a dry cough, which unfortunately often requires a change in therapy. ARBs are not associated with dry cough. Angioedema is very rare; however, patients started on ACE inhibitors or ARBs should be informed of the signs and symptoms that may present with angioedema. Inform patients that angioedema is unlikely, but if they experience swelling in their face (often including the eyelids) and/or extremities, they should discontinue treatment and seek medical attention immediately.18
Thiazide vs. Loop Diuretics: For patients without proteinuria, a preferred first-line therapy has not been clearly established, and other agents, such as a thiazide, may be considered. Patients with CKD and HTN often experience fluid retention or fluid overload. As a result, diuretics are often necessary in their treatment regimen. Thiazides are recommended for patients with CKD stages 1 to 3 (GFR ≥30 mL/min), and have been established as effective agents for BP and CVD risk reduction.22 Loop diuretics are recommended for patients with CKD stage 4 or 5 (GFR <30 mL/min), as they have been shown to be more effective in reducing extracellular fluid volume in patients with severely reduced GFR.18 However, the long-term effect of loop diuretics on cardiovascular outcomes has not been clearly established.23
Thiazide diuretics (chlorthalidone, hydrochlorothiazide) and loop diuretics (bumetanide, furosemide, torsemide) all cause hyperuricemia (increased urination). This increase in fluid loss may lead to electrolyte imbalance. It is important for patients on these agents to have their electrolytes monitored to ensure they do not experience electrolyte abnormalities such as hyperkalemia or hypomagnesemia. Orthostatic hypotension may occur in response to any antihypertensive agents; however, it is common with diuretics. It is important to counsel patients initiating diuretic therapy on the need to rise slowly from a sitting or lying-down position.24,25
Calcium Channel Blockers: Calcium channel blockers (CCBs) are considered second- or third-line therapy in the treatment of HTN in patients with CKD.8,9 While there may be no difference in the effect on BP lowering between nondihydropyridine CCBs (ND-CCBs; e.g., diltiazem, verapamil) and dihydropyridine CCBs (e.g., amlodipine, nifedipine), ND-CCBs have been shown to significantly reduce proteinuria either when used alone or in combination with an ACE inhibitor or an ARB.26 Because of their potential to reduce proteinuria, in addition to their antihypertensive effects, ND-CCBs should be considered as second- or third-line therapy in patients with diabetic CKD or nondiabetic CKD with proteinuria. Dihydropyridine CCBs can be used as second-line agents in patients with nondiabetic CKD without proteinuria. Common adverse effects include edema and constipation with ND-CCBs (especially verapamil) and flushing and peripheral edema with dihydropyridine agents.18
Aldosterone Antagonists: Aldosterone plays a severely deleterious role in the progression of CKD. Aldosterone receptor antagonists (e.g., spironolactone, eplerenone) may have a place in the role of CKD therapy when BP goals have not been achieved with first- and second-line therapy. These agents have shown in human trials to provide a reduction in proteinuria when added to an ACE inhibitor or ARB.27,28
Aldosterone antagonists are potassium-sparing diuretics, which increase the risk for hyperkalemia, particularly if taken with an ACE-inhibitor or ARB. It is important for patients initiated on potassium-sparing diuretics to have their potassium levels checked to ensure they do not experience electrolyte abnormalities. Symptoms of hyperkalemia include heart arrhythmia and severe muscle weakness. Unfortunately, hyperkalemia may present asymptomatically, which underscores the importance of monitoring.18,27
Renin Inhibitor: Aliskiren is the only renin inhibitor currently available on the market. It is indicated for the treatment of HTN as monotherapy or as combination therapy with valsartan. Recent data from the ALTITUDE trial have lead to the contraindication of its use with ACE inhibitors or ARBs in patients with diabetes or renal impairment (GFR <60 mL/min) due to the increased risk for renal impairment, hypotension, and hyperkalemia.29 Aliskiren can be considered if the patient cannot take an ACE inhibitor or an ARB; however, its use cannot be recommended in patients with stage 4 or 5 renal failure.30
Beta-Blockers: Data that evaluate the effect of beta-blockers on the progression of CKD and proteinuria are limited.27 While beta-blockers are not included in TABLE 2, these agents can be considered as second- or third-line therapy if the patient has a compelling indication for a beta-blocker such as coronary artery disease or chronic heart failure.18
Nonpharmacologic Recommendations
Chronotherapy: This type of therapy takes into consideration circadian BP patterns, and institutes administration of antihypertensive medication in respect to the daily patterns, moving away from administration of all antihypertensive medications in the morning. Trials have demonstrated improved 24-hour BP control in patients administering CCBs in the evening rather than in the morning.31,32 Additional studies have identified benefit from nighttime administration of other antihypertensive medications such as ACE inhibitors or ARBs.33,34 Chronotherapy may warrant some consideration for those unable to achieve their BP goal when administering all antihypertensive agents in the morning. If patients are on more than two antihypertensive agents, it may be appropriate to administer two agents in the morning and the additional agents in the evening.
Lifestyle Modification: Increased physical activity, weight loss, and dietary modifications are recommended for all patients with HTN.8 Lifestyle modification remains a critical component of therapy for HTN, regardless of whether patients require medications to achieve their BP goal. The Dietary Approaches to Stop Hypertension (DASH) diet emphasizes an increased consumption of fruits and vegetables, inclusion of low-fat dairy and lean protein, and a restriction of saturated fats; this meal plan has been shown to significantly lower systolic BP nearly equivalent to the reduction achieved by antihypertensive monotherapy.35 In addition, decreasing sodium and alcohol intake has been established as an effective intervention towards decreasing BP.8 Initiating these healthy dietary practices while increasing daily activity augments the benefit received from anti-hypertensive therapy and can play an essential role in achieving BP goals.
Summary
The interrelationship of CKD and HTN leads to further emphasis on the importance of achieving BP control and decreasing proteinuria, if present. Agents that reduce proteinuria in addition to BP are generally first line, but patients may often require three to four antihypertensive agents in order to achieve their goals and minimize their risk for CVD and ESRD. In addition, healthy lifestyle modifications should always be considered as a vital component of any antihypertensive therapy regimen.
REFERENCES
1. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038-2047.
2. Collins AJ, Foley RN, Chavers B, et al. U.S. renal data system 2011 annual data report. Am J Kidney Dis. 2012;59(suppl 1):evii.
3. Matsushita K, van der Velde M, Astor BC, et al.
Association of estimated glomerular filtration rate and albuminuria with
all-cause and cardiovascular mortality in general population cohorts: a
collaborative meta-analysis. Lancet. 2010;375:2073-2081.
4. Rashidi A, Sehgal AR, Rahman M, O’Connor AS. The case
for chronic kidney disease, diabetes mellitus, and myocardial infarction
being equivalent risk factors for cardiovascular mortality inpatients
older than 65 years. Am J Cardiol. 2008;102:1668-1673.
5. Rao MV, Qiu Y, Wang C, Bakris G. Hypertension and CKD:
Kidney Early Evaluation Program (KEEP) and National Health and Nutrition
Examination Survey (NHANES), 1999-2004. Am J Kidney Dis. 2008;51(suppl 2):S30-S37.
6. Botdorf J, Chaudhary K, Whaley-Connell A. Hypertension in cardiovascular and kidney disease. Cardiorenal Med. 2011;1:183-192.
7. Segura J, Ruilope L. Hypertension in moderate-to-severe nondiabetic CKD patients. Adv Chronic Kidney Dis. 2011;18:23-27.
8. Chobanian AV, Bakris GL, Black HR, et al. The seventh
report of the Joint National Committee on Prevention, Detection,
Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
9. National Kidney Foundation. K/DOQI clinical practice
guidelines for chronic kidney disease: evaluation, classification, and
stratification. Am J Kidney Dis. 2002;39(suppl 1):S1-S266.
10. American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care. 2012;35(suppl 1):S1-S63.
11. Bakris GL, Williams M, Dworkin L, et al. Preserving
renal function in adults with hypertension and diabetes: a consensus
approach. National Kidney Foundation Hypertension and Diabetes Executive
Committees Working Group. Am J Kidney Dis. 2000;36:646-661.
12. Keane WF, Eknoyan G. Proteinuria, albuminuria, risk,
assessment, detection, elimination (PARADE): a position paper for the
National Kidney Foundation. Am J Kidney Dis. 1999;33:1004-1010.
13. Yoshioka T, Rennke HG, Salant DJ, et al. Role of
abnormally high transmural pressure in the permselectivity defect of
glomerular capillary wall: a study in early passive Heymann nephritis. Circ Res. 1987:61:531-538.
14. Sarnak M, Levey A, Schoolwerth A, et al. Kidney
disease as a risk factor for the development of cardiovascular disease: a
statement from the American Heart Association Councils on Kidney in
Cardiovascular Disease, High Blood Pressure Research, Clinical
Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154-2169.
15. Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic
review: blood pressure target in chronic kidney disease and proteinuria
as an effect modifier. Ann Intern Med. 2011;154:541-548.
16. Cushman C, Evan GW Byington RP, et al. The ACCORD
study group. Intensive blood-pressure control in type 2 diabetes
mellitus. N Engl J Med. 2010;363:1575-1585.
17. Brenner BM, Cooper ME, De Zeeuw D, et al. Effects of
losartan on renal and cardiovascular outcomes in type 2 diabetes and
nephropathy. N Engl J Med. 2001;345:861-869.
18. Kidney Disease Outcomes Quality Initiative (K/DOQI).
K/DOQI clinical practice guidelines on hypertension and antihypertensive
agents in chronic kidney disease. Am J Kidney Dis. 2004;43(suppl 1):S1-S290.
19. Remuzzi G, Chiurchiu C, Ruggenenti P. Proteinuria predicting outcome in renal disease: nondiabetic nephropathies (REIN). Kidney Int Suppl. 2004;66:S90-S96.
20. Sharma P, Blackburn RC, Parke CL, et al.
Angiotensin-converting enzyme inhibitors and angiotensin receptor
blockers for adults with early (stage 1 to 3) non-diabetic chronic
kidney disease. Cochrane Database Syst Rev. 2011;(10):CD007751.
21. van Vark LC, Bertrand M, Akkerhuis KM, et al.
Angiotensin-converting enzyme inhibitors reduce mortality in
hypertension: a meta-analysis of randomized clinical trials of
renin-angiotensin-aldosterone system inhibitors involving 158,998
patients. Eur Heart J. 2012 Apr 17; Epub ahead of print.
22. Major outcomes in high-risk hypertensive patients
randomized to angiotensin-converting enzyme inhibitor or calcium channel
blocker vs. diuretic: the Antihypertensive and Lipid-Lowering Treatment
to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981-2997.
23. Musini VM, Wright JM, Bassett K, Jauca CD. Blood pressure lowering efficacy of loop diuretics for primary hypertension. Cochrane Database Syst Rev. 2009;(4):CD003825.
24. Microzide (hydrochlorothiazide oral capsules) package insert. Watson Pharma, Inc: Morristown, NJ; 2011.
25. Chlorthalidone oral tablets package insert. Mylan Pharmaceuticals, Inc: Morgantown, WV; 2006.
26. Bakris GL, Weir MR, Secic M, et al. Differential
effects of calcium antagonist subclasses on markers of nephropathy
progression. Kidney Int. 2004;65:1991-2002.
27. Navaneethan SD, Nigwekar SU, Sehgal AR, et al.
Aldosterone antagonists for preventing the progression of chronic kidney
disease. Clin J Am Soc Nephrol. 2009;4:542-551.
28. Mehdi UF, Adams-Huet B, Raskin P, et al. Addition of
angiotensin receptor blockade or mineralocorticoid antagonism to maximal
angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol. 2009;20:2641-2650.
29. Aliskiren-containing medications: drug safety communication—new warning and contraindication. MedWatch.
April 20, 2012.
www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm301120.htm?source=govdelivery;4/20/2012.
Accessed May 1, 2012.
30. Trimarchi H. Role of aliskiren in blood pressure control and renoprotection. Int J Nephrol Renovasc Dis. 2011;4:41-48.
31. Portaluppi F, Vergnani L, Manfredini R, et al.
Time-dependent effect of isradipine on the nocturnal hypertension in
chronic renal failure. Am J Hypertens. 1995;8:719-726.
32. Hermida RC, Calvo C, Ayala DE, et al. Dose and
administration-time dependent effects of nifedipine GITS on ambulatory
blood pressure in hypertensive subjects. Chronobiol Int. 2007;24:471-493.
33. Hermida RC, Ayala DE. Chronotherapy with the
angiotensin-converting enzyme inhibitor ramipril in essential
hypertension: improved blood pressure control with bedtime dosing. Hypertension. 2009;54:40-46.
34. Hermida RC, Calvo C, Ayala DE, et al. Administration
time-dependent effects of valsartan on ambulatory blood pressure in
hypertensive subjects. Hypertension. 2003;42:283-290.
35. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on
blood pressure of reduced dietary sodium and the Dietary Approaches to
Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group.
N Engl J Med. 2001;344:3-10.
To comment on this article, contact rdavidson@uspharmacist.com.






