US Pharm. 2013;38(3):22-26.
ABSTRACT: Gout is a rheumatic disease that results from an excess body burden of uric acid, or hyperuricemia, which commonly manifests as recurrent episodes of acute joint pain and inflammation secondary to the deposition of monosodium urate crystals, or tophi, in the synovial fluid and lining. Hyperuricemia is caused by an increased production or a decreased excretion of uric acid, or both. Treatment of gout involves managing hyperuricemia with urate-lowering therapy (i.e., diet, lifestyle, pharmacologic agents) and of acute gouty arthritis with colchicine, nonsteroidal anti-inflammatory drugs, and/or corticosteroids. Pharmacists play an integral role in patient education and improving the care of patients with gout.
Gout is a rheumatic disease that results from an excess body burden of uric acid, or hyperuricemia, which is variably defined as a serum urate concentration >6.8 or 7 mg/dL.1 Gout commonly manifests as recurrent episodes of acute joint pain and inflammation secondary to the deposition of monosodium urate (MSU) crystals (tophi) in the synovial fluid and lining. Furthermore, renal involvement of gout due to MSU deposition in the urinary tract can result in urolithiasis and urinary obstruction.2,3 Hyperuricemia is caused by an increased production or a decreased excretion of uric acid, or both. Underexcretion, the most common cause, is thought to account for 80% to 90% of hyperuricemia.4 Although the risk of developing gout increases with the chronicity and increased concentrations of uric acid (>9 mg/dL), not all patients with hyperuricemia will develop gout.2,3
Gout is the most common rheumatic disease of adulthood, with a self-reported prevalence of more than 8 million cases in the United States, affecting 3.9% of adults, with a male-to-female ratio of 3:1.5 However, at an older age the incidence of gout in women approaches the incidence in men.6
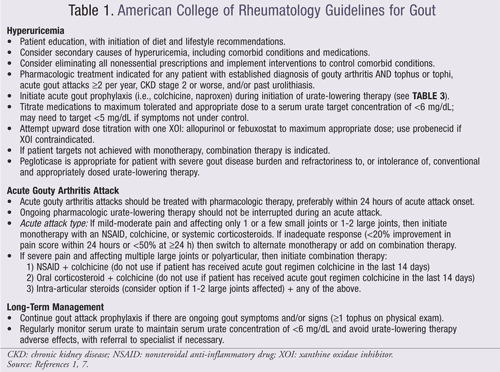
New gout guidelines from the American College of Rheumatology (ACR) have been recently published focusing on the use of urate-lowering therapy (ULT), analgesic and anti-inflammatory medications for the management of acute gouty arthritis, and drug prophylaxis of acute attacks (TABLE 1).1,7
Risk Factors
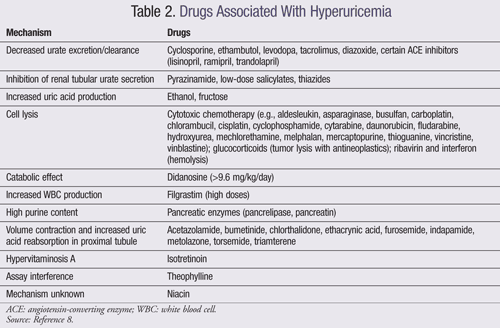
The prevalence of gout is increasing, mediated by an increased prevalence of comorbidities that promote hyperuricemia such as hypertension, obesity, metabolic syndrome, diabetes, and chronic kidney disease (CKD). The consumption of certain foods and beverages has been associated with increased incidence of gout, including purines found in meat and seafood, ethanol, soft drinks, and fructose. Additionally, certain drugs can also cause hyperuricemia (TABLE 2).1,2,8
Symptoms
There are a number of published criteria to diagnose gout.9,10 Several other conditions mimic the presentation of gout, including other crystal-induced arthritis, trauma, and septic joint. A typical presentation of gout includes severe pain, swelling, tenderness, fever, flulike symptoms, and often erythema of the joint that reaches peak intensity within 6 to 12 hours.11,12 The attack is most common at the first metatarsophalangeal joint or great toe (also known as podagra), or at the knee. Several procedures and laboratory testing such as joint aspiration, radiography, serum uric acid concentrations, and kidney uric acid excretion may be considered if gout is suspected.2
Nonpharmacologic Treatment
Nonpharmacologic treatment includes diet, lifestyle modifications, and general health promotion to control comorbid conditions including obesity, metabolic syndrome, hypertension, hyperlipidemia, diabetes, and CKD.1,10,13 There is a paucity of rigorous data determining the true impact of diet and lifestyle intervention in gout patients; however, smaller studies have been published,14-18 and a systematic review is currently under way.19
Lifestyle recommendations include regular exercise to achieve physical fitness, weight loss to achieve a body mass index (BMI) that promotes general health, maintaining proper diet (i.e., low-fat or nonfat dairy products, vegetables), hydration, and smoking cessation. Foods that patients should avoid include organ meats high in purine content (e.g., liver, kidney), high-fructose corn syrup–sweetened beverages, alcohol overuse (i.e., >2 drinks/day in males and >1 drink/day in females), and alcohol consumption during gouty attacks. Servings of red meats (e.g., beef, lamb, pork), seafood (e.g., canned fish, shellfish), fruit juices, table sugar, and salt should be restricted.1
Many drugs may elevate serum urate concentrations; thus, it is recommended that if such a drug is determined nonessential, it be discontinued once a diagnosis of gout is obtained. In some circumstances, and if dictated by the presence of comorbid conditions, some medications that could elevate serum urate concentrations may still need to be continued.1
Pharmacologic Treatment
Urate-Lowering Therapies: The purpose of lowering serum urate concentrations is to prevent acute gout attacks and the development of tophi. According to the ACR, pharmacologic treatment of hyperuricemia is indicated for any patient with an established diagnosis of active gouty arthritis (TABLE 1).1,7 ULT may be initiated during a gouty attack provided that effective anti-inflammatory management has been instituted. Furthermore, prophylaxis is recommended to reduce the risk of gout attacks associated with the initiation of ULTs and should be continued for 6 months (TABLE 3).2 Proper dose titration of medications and monitoring (every 2-5 weeks) of urate concentrations, renal function, and adverse effects of treatment are necessary. A minimum serum urate target concentration of <6 mg/dL should be obtained with therapy. Three classes of drugs are approved for lowering urate concentrations: xanthine oxidase inhibitors (XOIs), uricosuric agents, and uricase agents.
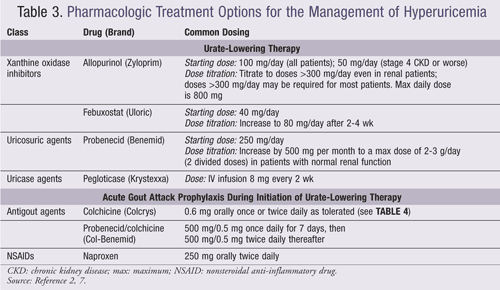
XOIs (e.g., allopurinol, febuxostat) block the synthesis of uric acid. Allopurinol is recommended for monotherapy and should be titrated over 2 to 5 weeks to achieve the serum urate target or the maximum tolerated doses. Allopurinol should be initiated at low doses to reduce the risk of hypersensitivity reactions and acute gout attack.1,10,20 Allopurinol-induced hypersensitivity reactions are rare but carry substantial morbidity and mortality rates of up to 25%.21,22 Allopurinol hypersensitivity reactions may be mitigated by screening for the presence of the HLA-B*5801 allele prior to initiation of therapy in susceptible patients (i.e., Koreans with CKD stage 3 or worse, Han Chinese or Thai descendants).23-25 Success in achieving target serum concentrations most often occurs when daily doses of allopurinol are titrated above an average dose of 300 mg/daily.26,27
Febuxostat, an XOI, is approved for the treatment of hyperuricemia in patients with gout and is also recommended for first-line treatment.1,28 In two randomized, controlled trials, all doses of febuxostat were more effective than allopurinol at usual doses in lowering serum urate concentrations to <6 mg/dL in both patients with normal kidney function and renally impaired patients.29,30 Patients frequently complained of acute gout attack with high-dose febuxostat.29 The major limitation of both trials is that allopurinol doses were not titrated higher than 300 mg, which is often necessary and more effective in obtaining target urate concentrations.25,26 Febuxostat can be substituted for allopurinol or vice versa in the event of drug intolerance or adverse events and after initial failure of upward dose titration.1 The safety of febuxostat in patients with allopurinol hypersensitivity has not been studied rigorously; however, a recent retrospective study revealed that it may be safe to use.31
Uricosuric agents (e.g., probenecid) reduce the serum urate concentration via blockade of renal tubular urate reabsorption. Probenecid is contraindicated in patients with a history of urolithiasis or presence of uric acid overproduction. To mitigate the risk of nephrolithiasis, it is recommended that patients be screened for urine uric acid. Patients should be encouraged to increase fluid intake and urine alkalinization with potassium citrate may be considered.32 Uricosuric agents may be added to XOIs to achieve target serum urate concentrations. Fenofibrate and losartan have shown to possess uricosuric effects. Although studies are limited to small trials and case reports,33-36 fenofibrate or losartan may be considered as adjuvant therapy in gouty patients on allopurinol and hyperlipidemia or hypertension, respectively.37 Ingestion of vitamin C 500 mg has also been associated with minimal lowering of serum urate concentrations, which may have little clinical significance.38
Uricase agents (e.g., pegloticase) convert uric acid into soluble allantoin. Pegloticase is approved for chronic gout that is refractory to conventional treatments.1,39 Treatment with pegloticase resulted in successful reduction of serum urate to <6 mg/dL in a phase II randomized study, with gouty flares being the most common adverse effect in this trial.40 Other cited adverse effects include infusion site reactions and anaphylaxis.39
Acute Gouty Arthritis
The diagnosis and treatment of gouty arthritis is determined by the severity of pain, duration of attack, and extent of joint involvement. Pain assessment is commonly based on a visual analogue scale of 0 to 10 where ≤4 is mild, 5 to 6 is moderate, and ≥7 is severe. The duration of gouty arthritis is measured from the onset of gouty pain where <12 hours is early, 12 to 36 hours is well established, and >36 hours is late. The extent of an acute gouty arthritis attack is determined by the number of active joints. Gouty arthritis should be treated with pharmacologic therapy within 24 hours of attack and for up 7 to 10 days.2,7 ULTs should be continued during an acute attack.7 Monotherapy with nonsteroidal anti-inflammatory drugs (NSAIDs), systemic corticosteroids, or colchicine is recommended for mild-to-moderate pain where only one or a few small joints are affected. Combination therapy is recommended for polyarticular attacks or an attack affecting multiple large joints that induce severe pain (TABLE 4).7
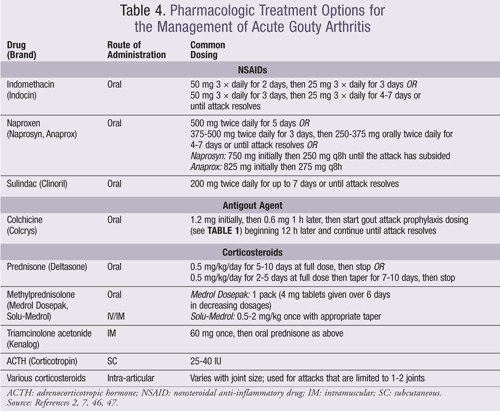
Colchicine exerts its effects by reducing lactic acid production by leukocytes, which in turn decreases uric acid deposition and reduces phagocytosis, with abatement of the inflammatory response. Although an older drug, colchicine just recently obtained an FDA indication for use in patients with acute gout.41 Colchicine should be instituted only in early or well-established gouty attacks. In a recent randomized trial, low-dose colchicine (1.8 mg over 1 h) yielded both maximum plasma concentration and early gout-flare efficacy comparable to high-dose colchicine (4.8 over 6 h), with a safety profile indistinguishable from that of placebo.42
NSAIDs inhibit the cyclooxygenase (COX) enzymes, which are involved in the inflammatory process and prostaglandin production. The three NSAIDs that are FDA approved for the treatment of acute gout are naproxen, indomethacin, and sulindac.7 Celecoxib, the only COX-2 inhibitor currently available in the U.S., does not have an indication for acute gout.43 However, celecoxib has been shown to effectively treat acute gout at high doses (800 mg/day) in a randomized study,44 although these doses exceed the recommended maximum daily doses.43
Corticosteroids are recommended by the ACR for monotherapy, but these agents are generally reserved for patients who cannot tolerate either colchicine or NSAIDs due to their systemic adverse effects.7 Although the guidelines do not explicitly recommend depo formulations, these agents have been used; however, dosing is ambiguous.45 When selecting corticosteroids as the initial therapy, joint involvement needs to be considered (TABLE 4).7
Role of the Pharmacist
Pharmacists play an integral role in patient education and improving the care of patients with gout. They can help patients improve adherence to pharmacologic and nonpharmacologic therapies. Pharmacists should emphasize lifestyle modifications and diet such as managing weight, limiting meat and seafood intake, and minimizing alcohol intake. Pharmacists should also educate patients about their gout medications, including appropriate dosing, duration of therapy, adverse effects, contraindications, and drug interactions.
REFERENCES
1. Khanna D, Fitzgerald JD, Khanna PP, et al; American
College of Rheumatology. 2012 American College of Rheumatology
guidelines for management of gout. Part 1: Systematic nonpharmacologic
and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431-1446.
2. Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364:443-452.
3. Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30-38.
4. Centers for Disease Control and Prevention (CDC). Gout. www.cdc.gov/arthritis/basics/gout.htm. Accessed January 2, 2013.
5. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and
hyperuricemia in the US general population: the National Health and
Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136-3141.
6. Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;75(suppl 5):S9-S12.
7. Khanna D, Khanna PP, Fitzgerald JD, et al; American
College of Rheumatology. 2012 American College of Rheumatology
guidelines for management of gout. Part 2: Therapy and antiinflammatory
prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64:1447-1461.
8. Bohan KH. Gout and hyperuricemia. In: Alldredge BK, Corelli RL, Ernst ME, et al, eds. Koda-Kimble and Young’s Applied Therapeutics: The Clinical Use of Drugs. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013:1049.
9. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895-900.
10. Zhang W, Doherty M, Bardin T, et al; EULAR Standing
Committee for International Clinical Studies Including Therapeutics.
EULAR evidence-based recommendations for gout. Part II: Management.
Report of a task force of the EULAR Standing Committee for International
Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312-1324.
11. Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med. 2008;75(suppl 5):S5-S8.
12. Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76:801-808.
13. Jordan KM, Cameron JS, Snaith M, et al. British
Society for Rheumatology and British Health Professionals in
Rheumatology Standards, Guidelines and Audit Working Group (SGAWG).
British Society for Rheumatology and British Health Professionals in
Rheumatology guideline for the management of gout. Rheumatology (Oxford). 2007;46:1372-1374.
14. Choi HK, Soriano LC, Zhang Y, Rodriguez LA.
Antihypertensive drugs and risk of incident gout among patients with
hypertension: population based case-control study. BMJ. 2012;344:d8190.
15. Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336:309-312.
16. Choi HK, Atkinson K, Karlson EW, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350:1093-1103.
17. Choi HK, Atkinson K, Karlson EW, et al. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363:1277-1281.
18. Choi HK, Willett W, Curhan G. Fructose-rich beverages and risk of gout in women. JAMA. 2010;304:2270-2278.
19. Moi JH, Sriranganathan MK, Edwards CJ, Buchbinder R. Lifestyle interventions for chronic gout. Cochrane Database Syst Rev. 2012;(8):CD010039.
20. Stamp LK, Taylor WJ, Jones PB, et al. Starting dose is
a risk factor for allopurinol hypersensitivity syndrome: a proposed
safe starting dose of allopurinol. Arthritis Rheum. 2012;64:2529-2536.
21. Lupton GP, Odom RB. The allopurinol hypersensitivity syndrome. J Am Acad Dermatol. 1979;1:365-374.
22. Arellano F, Sacristán JA. Allopurinol hypersensitivity syndrome: a review. Ann Pharmacother. 1993;27:337-343.
23. Zineh I, Mummaneni P, Lyndly J, et al. Allopurinol pharmacogenetics: assessment of potential clinical usefulness. Pharmacogenomics. 2011;12:1741-1749.
24. Somkrua R, Eickman EE, Saokaew S, et al. Association
of HLA-B*5801 allele and allopurinol-induced Stevens Johnson syndrome
and toxic epidermal necrolysis: a systematic review and meta-analysis. BMC Med Genet. 2011;12:118.
25. Lee MH, Stocker SL, Anderson J, et al. Initiating allopurinol therapy: do we need to know the patient’s HLA status? Intern Med J. 2011;42:411-416.
26. Reinders MK, Haagsma C, Jansen TL, et al. A randomised
controlled trial on the efficacy and tolerability with dose escalation
of allopurinol 300-600 mg/day versus benzbromarone 100-200 mg/day in
patients with gout. Ann Rheum Dis. 2009;68:892-897.
27. Stamp LK, O’Donnell JL, Zhang M, et al. Using
allopurinol above the dose based on creatinine clearance is effective
and safe in patients with chronic gout, including those with renal
impairment. Arthritis Rheum. 2011;63:412-421.
28. Uloric (febuxostat) package insert. Deerfield, IL: Takeda Pharmaceuticals America, Inc; November 2012.
29. Becker MA, Schumacher HR Jr, Wortmann RL, et al.
Febuxostat compared with allopurinol in patients with hyperuricemia and
gout. N Engl J Med. 2005;353:2450-2461.
30. Becker MA, Schumacher HR, Espinoza LR, et al. The
urate-lowering efficacy and safety of febuxostat in the treatment of the
hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12:R63.
31. Chohan S. Safety and efficacy of febuxostat treatment in subjects with gout and severe allopurinol adverse reactions. J Rheumatol. 2011;38:1957-1959.
32. Perez-Ruiz F, Hernandez-Baldizon S, Herrero-Beites AM,
et al. Risk factors associated with renal lithiasis during uricosuric
treatment of hyperuricemia in patients with gout. Arthritis Care Res (Hoboken). 2010;62:1299-1305.
33. Hepburn AL, Kaye SA, Feher MD. Fenofibrate: a new treatment for hyperuricaemia and gout? Ann Rheum Dis. 2001;60:984-986.
34. Würzner G, Gerster JC, Chiolero A, et al. Comparative
effects of losartan and irbesartan on serum uric acid in hypertensive
patients with hyperuricaemia and gout. J Hypertens. 2001;19:1855-1860.
35. Takahashi S, Moriwaki Y, Yamamoto T, et al. Effects of
combination treatment using anti-hyperuricaemic agents with fenofibrate
and/or losartan on uric acid metabolism. Ann Rheum Dis. 2003;62:572-575.
36. Hepburn AL, Kaye SA, Feher MD. Long-term remission from gout associated with fenofibrate therapy. Clin Rheumatol. 2003;22:73-76.
37. Perez-Ruiz F. New treatments for gout. Joint Bone Spine. 2007;74:313-315.
38. Huang HY, Appel LJ, Choi MJ, et al. The effects of
vitamin C supplementation on serum concentrations of uric acid: results
of a randomized controlled trial. Arthritis Rheum. 2005;52:1843-1847.
39. Krystexxa (pegloticase) package insert. East Brunswick, NJ: Savient Pharmaceuticals, Inc; 2010.
40. Sundy JS, Becker MA, Baraf HS, et al; Pegloticase
Phase 2 Study Investigators. Reduction of plasma urate concentrations
following treatment with multiple doses of pegloticase (polyethylene
glycol-conjugated uricase) in patients with treatment-failure gout:
results of a phase II randomized study. Arthritis Rheum. 2008;58:2882-2889.
41. Colcrys (colchicine) package insert. Deerfield, IL: Takeda Pharmaceuticals America, Inc; June 2012.
42. Terkeltaub RA, Furst DE, Bennett K, et al. High versus
low dosing of oral colchicine for early acute gout flare:
twenty-four-hour outcome of the first multicenter, randomized,
double-blind, placebo-controlled, parallel-group, dose-comparison
colchicine study. Arthritis Rheum. 2010;62:1060-1068.
43. Celebrex (celecoxib) package insert. New York, NY: Pfizer Inc; January 2013.
44. Schumacher HR, Berger MF, Li-Yu J, et al. Efficacy and
tolerability of celecoxib in the treatment of acute gouty arthritis: a
randomized controlled trial. J Rheumatol. 2012;39:1859-1866.
45. Depo-Medrol (methylprednisolone acetate injectable suspension, USP) package insert. New York, NY: Pfizer Inc; January 2012.
46. Naprosyn (naproxen) package insert. Nutley, NJ: Roche Laboratories, Inc; 2008.
47. Anaprox (naproxen) package insert. North Dayton, NJ: Aurobindo Pharma USA, Inc; 2011.
To comment on this article, contact rdavidson@uspharmacist.com.





