US Pharm. 2013;38(6):HS13-HS16.
ABSTRACT: There are numerous wound dressings and management techniques available today. These include absorbent dressings, films, hydrocolloids, hydrogels, foams, antibiotic dressings, larvae (maggot) therapy, and vacuum-assisted wound closure. The challenge lies not only in choosing the correct dressing, but also in using the chosen technique properly. This involves careful assessment of the wound, taking into account its size, the exudate, and the patient’s preferences. Health care professionals require basic knowledge of dressings for correct application, and the wound should be monitored closely to ensure effective healing.
A wound can be described as any process that leads to the disruption of the normal architecture and function of the tissue.1 It may be a closed wound such as a bruise or sprain or an open wound such as an abrasion, laceration, avulsion, or ballistic or surgical wound.2 This article discusses the management of wounds that cause a break in the skin’s integrity, i.e., open wounds.
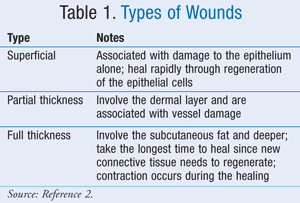
A wound needs to be thoroughly assessed before an appropriate dressing regimen can be drawn up. The wound is often classified based upon the number of skin layers affected (TABLE 1).2
Wound Healing
Wound healing is a highly complex process that results in the restoration of cell structures and tissue layers after an injury.3 It involves interdependent and overlapping cellular, physiological, biochemical, and molecular processes that can be divided into three phases: inflammation, proliferation, and maturation.2,3 An acute wound will usually heal within a few weeks, whereas chronic wounds take longer than 6 weeks to heal.3
Inflammation: Part of the body’s normal defense mechanism, the inflammatory phase is essential to the healing process and occurs within seconds of injury. This stage can last up to 3 days and is typically associated with redness, heat, swelling, and pain.4
There is immediate vasoconstriction of the damaged blood vessels and coagulation to limit blood loss.3 Platelets are attracted to the damaged blood vessels and the coagulation cascade is initiated, leading to a platelet plug that is later stabilized by fibrin.3 Following this, histamine and other chemical mediators are released from the dam-aged cells, resulting in vasodilation.4 Vasodilation releases growth factors and brings white blood cells (WBCs) to the area, leading to the release of other chemical mediators that are essential for wound healing.3 Some of these mediators increase capillary permeability, causing exudate to be released into the wound area.4
Exudate is a broad term used to describe the fluid that is produced by wounds following hemostasis. It consists primarily of water and may include proteins, electrolytes, nutrients, proteases, growth factors, WBCs, platelets, and inflammatory mediators.5 Healthy exudate is pale amber in color, odorless, and watery and is a sign of healing.5 Unhealthy wound exudate is associated with an excessive volume and/or an altered content. If left unmanaged, it can lead to periwound skin maceration, delayed healing, malodor, and infection.5,6 Neutrophils in the exudate serve to remove foreign material and dead or dying cells and attract macrophages to the area.3
Proliferation: Also known as the fibroblastic or regenerative phase, proliferation typically lasts 2 to 3 weeks.7 Granulation tissue composed of macrophages, fibroblasts, immature collagen, blood vessels, and ground substance is formed. Fibroblasts play two roles in the wound healing process: 1) they stimulate the production of collagen and elastin that increases the strength of the wound, and 2) they stimulate the growth of new blood vessels.3 As granulation tissue fills the wound site, the edges of the wound pull together, decreasing the surface of the wound. Epithelialization is the final step, whereby epithelial cells migrate from the wound edges and the wound is finally covered, resulting in the formation of a scar.7
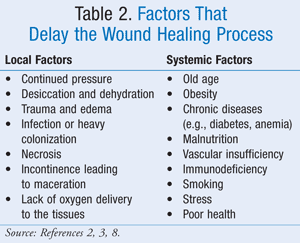
Maturation: The maturation phase can take anywhere from 3 weeks to 2 years.3 During this phase, collagen fibers cross link and reorganize, and the strength of the scar increases.2,7 Various local and systemic factors that can delay the wound healing process are listed in TABLE 2.2,3,8
Wound Dressings
Topical preparations for wound care have been used for centuries. Dressings play a major role in wound management and have developed greatly over the last 50 years from passive to more active types.9 The first dressings used in the 18th century were made from natural materials such as oakum. These dressings were absorbent but could not retain the exudate, caused infections, and adhered to the wound bed.5 This led to the development of Gamgee, an absorbent cotton wool core sandwiched between two layers of absorbent cotton gauze.5 In the 19th and 20th centuries, synthetic ingredients were increasingly used in the manufacture of dressings.7
Dressings protect the wound and keep it moist, thereby promoting healing.3 Only diabetic, dry, gangrenous toes require a moisture-free environment for effective healing. An ideal dressing should2,3,10,11:
• Maintain a moist environment
• Provide thermal insulation
• Be nonadherent
• Require infrequent changing
• Provide mechanical protection
• Be free from particulate contaminants
• Be safe to use (i.e., nontoxic, nonallergenic)
• Be conformable, moldable, and comfortable
• Have good absorption characteristics
• Be impermeable to microorganisms
• Be acceptable to the patient
• Be cost-effective and sterile
• Be available in a suitable range of forms and sizes.
Types of Dressings
While no single dressing meets all of the ideal criteria, there are a range of dressings that vary in shape and size, the level of exudate they can handle, and the anatomical site to which they can be applied. Selecting the most appropriate dressing for the wound in question is important.
Hydrocolloid, hydrogel, film, and foam dressings are able to handle large amounts of exudate and promote auto-debridement, while alginate and collagen-based dressing are mostly used for their ability to promote granulation tissue.9 Antibiotic-based dressings (e.g., neomycin, silver, iodine) have been developed in an attempt to avoid infections, one of the main causes of delayed wound healing.9 Such dressings, however, run the risk of allergy and resistance.9 Advances in wound management have led to the development of active dressings that facilitate the wound healing process as well as of techniques such as vacuum-assisted closure to remove cytokines and proteases.8 Primary dressings cover the wound directly, whereas secondary dressings are used to hold a primary dressing in place.3
Absorbent Dressings: Absorbent dressings have a capillary action that wicks away mild, moderate, or heavy drainage from wounds. These types of dressings help prevent the periwound area from becoming macerated.11 They may be used as primary or secondary dressings. Those without an adhesive border are usually changed once daily, whereas those with an adhesive border may be changed every other day.11
Hydrogels: Hydrogels consist of a matrix of hydrophilic polymers that swell in water but do not themselves dissolve.12,13 They contain about 60% to 90% water and are available as gels, sheets, and impregnated nonwoven dressings.3,11 Hydrogel wound dressings are effective, comfortable, easy to use, and cost-effective.14 Some hydrogel sheets have an adhesive border, but most require a secondary dressing.11
Hydrogels are useful for wounds with minimal or no exudate, painful wounds, burns, and skin tears, since they hydrate the wound surface and in some cases absorb excess exudate.13,14 This ability allows desloughing and debriding processes to necrotic and fibrotic tissue. Hydrogels are soothing, cooling, and may even reduce pain and thus are particularly useful when applied to radiation burns.3
On the downside, the high water content of hydrogels may lead to maceration around the wound area. It is therefore important that no more than the required amount of dressings be applied to the wound.3 Hydrogels contain propylene glycol, a chemical that is contraindicated before larvae therapy. Wounds that are to be treated using larvae therapy should therefore be free of hydrogels and thoroughly irrigated 24 hours before therapy. Hydrogel dressings are changed between 1 and 3 times a week.13,14
Hydrocolloids: Occlusive and adhesive wafer dressings are usually made by bonding sodium carboxy-methylcellulose, pectin, or gelatin to a carrier of semipermeable or foam material and are used to manage light-to-moderate amounts of wound exudate.12,13 Most hydrocolloids react with the wound exudate to form a gel-like covering that protects the wound bed and maintains a moist wound environment.13 In this way, they promote debridement of necrotic tissue.13
Hydrocolloids are available in sheets, pastes, and powders. The sheets are conformable for easy application and help reduce pain at the wound site; shaped sheets are available for awkward areas such as the heel and elbow.13 Pastes and powders tend to have a greater absorptive capacity than the sheets and are usually used to fill cavity wounds to the surface.3
Hydrocolloid wound dressings are best for granulating and epithelializing wounds with low-to-moderate amounts of exudate. They should not be used for dry wounds or exposed bone or muscle.3,13 Since they are occlusive, patients can shower while wearing a hydrocolloid dressing.12
These dressings break down to produce residue of various colors and a foul odor that may sometimes be mistaken for an infection.12 Hydrocolloids containing gelatin from pigs may not be acceptable to vegetarians and patients of some religious backgrounds.3 The frequency of changing the sheets depends upon the level of exudate and is usually between 5 and 7 days.
Alginates: Alginates are made of soft, nonwoven fibers derived from different varieties of seaweed and are available as pads, ropes, or ribbons.7,10 Alginates are either mannuronic or guluronic acid. Since they can absorb up to 20 times their own weight in fluid, they are particularly useful for highly exudating wounds.3 They absorb the wound exudate and form a gel-like covering over the wound, maintaining a moist wound environment. Unlike gauzes, alginates do not physically inhibit wound contraction.7
Alginates require a secondary dressing, and care should be taken to leave room for expansion when packing cavities.15 Alginates should not be used with hydrogels that will add fluid to the alginate and reduce its fluid-handling capacity.3
Film Dressings: Transparent adhesive film dressings are made of polyurethane and are semipermeable membrane dressings that are waterproof yet permeable to oxygen and water vapor.13 They maintain a moist wound environment and help prevent bacterial contamination.11,13 These films facilitate cellular migration and promote autolysis of necrotic tissue by trapping moisture at the wound surface.11
Film dressings are best for superficial wounds; wounds with light exudate; wounds on the elbows, heels, or flat surfaces; covering blisters; and for the retention of primary dressings, especially hydrocolloid and alginates, as they provide waterproof cover.16 Film dressings cannot be used for wounds with moderate-to-heavy exudate and must be selected carefully, as some newer films are intended for IV sites and may dry up the wound. They are usually changed up to 3 times per week.13
Foam Dressings: Foam dressings are highly absorbent dressings usually made from a hydrophobic polyurethane or silicone foam.13 They are useful for heavily exudating wounds, particularly during the inflammatory phase following debridement and desloughing, when drainage is at its peak.13 Foams are also effective in the packing of deep cavity wounds to prevent premature closure while absorbing exudate and maintaining a moist environment, and in weeping ulcers such as in venous stasis.17
Foam dressings rarely adhere to the wound bed and are conformable and very comfortable to wear.3 They can be worn during bathing and can frequently be left undisturbed for 3 to 4 days.13
Protease-Modulating Dressings: Proteases (e.g., matrix metalloproteinases) are associated with angiogenesis and the natural debridement and cleansing of wounds.3 Matrix metalloproteinases degrade collagen and other components of the extracellular matrix and assist in tissue remodeling by allowing cells to move through the wound bed.8,18
Compression Dressings: Compression dressings are layered primary dressings that are used to apply compression in the treatment of venous ulceration.10 They vary in the amount of stretch they provide intrinsically and extrinsically, and correct application is essential for maximum effectiveness. They may consist of two or more of the following layers10:
• First layer: a natural wool layer, which is subcompression wadding bandage used to absorb exudate and redistribute the pressure around the ankle; applied in a loose spiral
• Second layer: a crepe bandage that increases absorbency and smoothes the orthopedic wool layer
• Third layer: a light compression bandage
• Fourth layer: an elastic cohesive bandage that keeps all the layers in place.
Antimicrobial Dressings: Dressings impregnated with antibacterials, such as silver or iodine, are targeted at preventing infections. Additionally, topical bacitracin, polymyxin B, neomycin, and their compounds may be formulated into an ointment. These dressings are soothing, comfortable to wear, lubricating, occlusive, and deliver the antibiotic directly to the wound. They are effective in limiting scab formation. Silver may be added to alginate, hydrofiber, hydrocolloid, foam, and activated charcoal dressings.19 Silver exerts its antibacterial effects by9:
• Interfering with bacterial electron transport
• Binding to DNA of bacteria and their spores, increasing the stability of the double helix and impairing replication
• Interacting with the bacterial cell membrane and damaging the structure
• Forming insoluble, metabolically ineffective compounds.
It is important that the minimum effective amount of silver be applied to reduce systemic absorption, as silver may be toxic.9 Some patients may find the gray discoloration, formed when the silver reacts with pollutants in the environment to form silver sulfide, unpleasant.9 Silver dressings are used for 2 weeks and then the wound is reassessed. They should not be used in patients receiving radiotherapy, x-rays, ultrasound, and diathermy.19 Since silver is inactivated by protein binding, dressings that release silver slowly need to be changed less frequently than those that immediately release silver.9
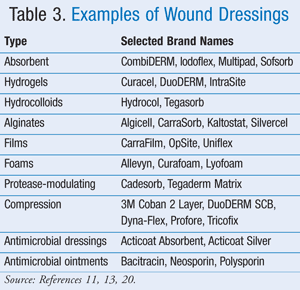
Examples of each of the dressing types reviewed are provided in TABLE 3.11,13,20
Other Wound Management Techniques
Vacuum-Assisted Wound Closure (VAC): This technique is a type of topical negative pressure therapy based on the theory that the application of negative pressure either continuously or intermittently can speed up the healing process.19 A vacuum is applied across the wound together with a specially designed foam dressing or moistened gauze connected to a vacuum machine by tubing. VAC is useful in enhancing blood flow, diminishing edema, limiting bacterial proliferation, and accelerating granulation and the formation of tissue in the wound.19
Larvae (Maggot) Therapy: Larvae therapy uses sterile fly larvae (maggots) to debride necrotic and sloughy wounds effectively and quickly, and in many cases it removes the need for surgical debridement.21,22 The maggots break down dead tissue by releasing proteolytic enzymes to digest it and promote the formation of granulation tissue.21 The maggots die very quickly and therefore should be applied the same day they are delivered. A nonocclusive dressing should be applied over the maggots, as they require oxygen to survive. The periwound area should be protected, because healthy skin can be damaged by the maggots.21
Larvae therapy can be used for purulent, sloughy wounds on the skin.23 The process takes about 3 to 5 days and requires close monitoring. The outer dressing should be changed as required to prevent the maggots from suffocating, and patients should be careful not to squash the maggots, especially at pressure sites.21
Larvae therapy is quick, efficient, and effective. Furthermore, it breaks down bacteria, thereby reducing malodor. This therapy, however, can be painful owing to the changing pH; patients may be prescribed a simple analgesic such as acetaminophen to ease the discomfort. Some patients and health care prescribers may be hesitant to use this therapy for fear of contact with maggots.21-23
Conclusion
With the large number of wound management products available in the market today, it is important that health care professionals be conversant with their function and application. It is well recognized that no one dressing has all the properties of an ideal dressing, but advances in technology have led to the development of techniques such as VAC and larvae therapy over and above the use of basic wound dressings.
REFERENCES
1. Cockbill S. Wounds, the healing process. Hosp Pharm. 2002;9:255-260.
2. Baranoski S, Ayello EA. Wound Care Essentials: Practice Principles. 3rd ed. New York, NY: Lippincott Williams & Wilkins; 2011.
3. Flynn J. Understanding chronic wound management: part 1. Pharm J. 2009;282:777-780.
4. Dealey C. The Care of Wounds: A Guide for Nurses. West Sussex, UK: Wiley-Blackwell; 2012.
5. Ovington LG. Advances in wound dressings. Clin Dermatol. 2007;25:33-38.
6. Sweeney IR, Miraftab M, Collyer G. A critical review of modern and emerging absorbent dressings used to treat exuding wounds. Int Wound J. 2012;9:601-612.
7. Hess CT. Wound Care. New York, NY: Lippincott Williams & Wilkins; 2005.
8. Rushton I. Understanding the role of proteases and pH in wound healing. Nurs Stand. 2007;21:68-72.
9. Leaper DJ. Silver dressings: their role in wound management. Int Wound J. 2006;3:282-294.
10. Morgan D. Wounds: what dressings should a formulary include? Hosp Pharm. 2002;9:261-266.
11. Lionelli GT, Lawrence WT. Wound dressings. Surg Clin North Am. 2003;83:617-638.
12. Jones V, Grey JE, Harding KG. Wound dressings. BMJ. 2006;332:777-780.
13. Kifer ZA. Fast Facts for Wound Care Nursing: Practical Wound Management in a Nutshell. New York, NY: Springer Publishing Co; 2011.
14. Eisenbud D, Hunter H, Kessler L, Zulkowski K. Hydrogel wound dressings: where do we stand in 2003? Ostomy Wound Manage. 2003;49:52-57.
15. Fletcher J. Understanding wound dressings: alginates. Nurs Times. 2005;101:53-54.
16. Young T. When to use film dressings. Community Nurse. 1998;4:36-37.
17. Fletcher J. Understanding wound dressings: foam dressings. Nurs Times. 2005;101:50-51.
18. Casey G. Wound repair: advanced dressing materials. Nurs Stand. 2002;17:49-53.
19. Flynn J. Understanding chronic wound management: part II. Pharm J. 2009;283:41-44.
20. Bennett-Marsden M. How to select a wound dressing. Clin Pharm. 2010;2:363-365.
21. Bonn D. Maggot therapy: an alternative for wound infection. Lancet. 2000;356:1174.
22. Mumcuoglu KY. Clinical applications for maggots in wound care. Am J Clin Dermatol. 2001;2:219-227.
23. Thomas S. Use of maggots in the care of wounds. Hosp Pharm. 2002;9:267-271.
To comment on this article, contact rdavidson@uspharmacist.com.





