US Pharm. 2014;39(12):HS2-HS.
ABSTRACT: Atrial fibrillation (AF) that is refractory to medication therapy is often managed with catheter ablation. Complications of catheter ablation include esophageal damage (erosion, ulceration, or perforation) leading to atrioesophageal fistula (AEF), which can be life-threatening. Although the mechanisms of AEF are not fully understood, it has been postulated that acid reflux contributes to the progression. Therefore, treatment with gastrointestinal (GI) protective therapy, including proton pump inhibitors, histamine-2 receptor antagonists, and sucralfate for up to 4 weeks, has demonstrated some efficacy. The 2012 Catheter and Surgical Ablation of Atrial Fibrillation guidelines recommend this for all patients post AF ablation. Pharmacists should be aware of the unique role of GI protective therapy in this special population and be diligent in ensuring proper use of these agents through education.
Atrial fibrillation (AF) is a supraventricular arrhythmia that is commonly treated with pharmacologic therapy.1 However, some patients may be refractory to medications and are often managed with catheter ablation. Catheter ablation has been shown to improve the patient’s quality of life, decrease stroke and heart failure risk, and improve survival.1,2 Although the mechanism of focal firing is not completely understood, it is well accepted that triggers of AF can stem from the pulmonary veins and the posterior left atrium.1 One of the most common approaches to ablation is to isolate and target the pulmonary veins.
Background
Currently, there are two common methods employed for ablation therapy, radiofrequency (RF) and cryothermal (cryoablation); however, other technologies such as ultrasound and laser ablation systems are available. RF energy is the predominantly used source for catheter ablations. It conducts alternating electrical currents through myocardial tissue, creating heat energy with the intensity of heating and tissue destruction being proportional to the delivered power. Cryoablation is an alternative technique where freezing is used to disrupt membrane ion transport to result in local conduction block, and it is associated with a low complication rate.1,2 Complications of this procedure are rare but can include cardiac tamponade, thromboembolism, bleeding, pulmonary vein stenosis, vagal nerve injury, and esophageal damage (erosion, ulceration, or perforation).1-3
Anatomically, the esophagus descends posterior to the left atrium, and these anatomical features can be as close as a few millimeters. In light of this close proximity, there is potential to cause thermal damage to the esophagus. Esophageal injury has the potential to lead to more serious complications such as atrioesophageal fistula (AEF), where a dangerous perforation develops that forms a connection between the left atrium and the esophagus. Despite having a low incidence (0.10%-0.25%), it is associated with a high morbidity and mortality rate of >80%.1,3 The exact mechanism of ulceration and progression to AEF is unclear; possible causes include direct thermal injury, acid reflux, and ischemic injury.1,2 Symptoms of AEF such as fevers, chills, cerebrovascular accidents, septic emboli, and death typically occur within 4 weeks of ablation.1,4,5
Different approaches have been executed to decrease the risk of developing the potentially fatal complication associated with AEF. One practice is to perform luminal esophageal temperature (LET) monitoring to lower the amount of thermal injury to the patient. LET monitoring involves using a temperature probe that is placed in the esophagus to help gauge high temperatures warranting a decrease in energy and/or a shorter duration of exposure.1-3,6
Another practice is the use of pharmacologic therapy such as proton pump inhibitors (PPIs) and other gastrointestinal (GI) protective agents.1 The potential usefulness of these agents is based on the theory that patients who develop esophageal ulceration are at risk of progression to AEF when GI reflux disease is present. Ablation can also cause vagal nerve damage, which may lead to relaxation of the lower esophageal sphincter (LES) and cause gastroparesis. This can lead to further erosion of esophageal ulcers through acid reflux, and is another potential area where GI protective agents can show benefit.1,7 According to the 2012 Catheter and Surgical Ablation of Atrial Fibrillation guidelines, PPIs or histamine-2 receptor antagonists (H2RAs) are routinely prescribed for 1 to 4 weeks following ablation. This, however, is based solely on observation since there are no data from randomized controlled trials to show that this practice reduces the incidence of developing AEF.1
Pharmacologic Therapies
Drugs commonly used to prevent the development of AEF are discussed below and summarized in TABLE 1.8-10
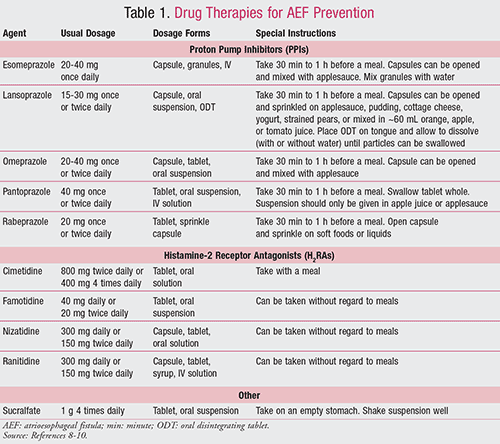
Proton Pump Inhibitors: PPIs are agents that diminish the normal physiological production of acid in the stomach via the H+, K+-ATPase pumps (i.e., proton pumps) by 80% to 95%. They irreversibly inactivate the proton pumps by binding covalently with sulfhydryl groups of cysteine. Acid release from the proton pumps is therefore halted and resumes once new pumps are formed. PPIs provide acid suppression up to 24 to 48 hours. Three to 4 days of daily therapy is necessary before full benefit is seen from these agents, due to the fact that not all pumps are inactivated when a first dose is given.8,9
Food decreases bioavailability by 50%; therefore, PPIs should be administered on an empty stomach. Only 10% of the pumps are active during a fasting state; hence, these agents should be given approximately 30 minutes to 1 hour prior to meals so the drug’s peak concentration is correlated with the peak proton pump activity during the fed state.8,9
Adverse events are rare but can include diarrhea, abdominal pain, and headache. Because of their acid-suppressive properties, PPIs have been associated with an increased risk of Clostridium difficile infection and respiratory infections such as pneumonia. Additionally, PPIs are associated with hypomagnesemia and osteoporosis due to the diminished absorption of minerals.8,9
There are many potential drug interactions associated with PPIs. PPIs raise the gastric pH, which inhibits the absorption of drugs that require an acidic environment (e.g., ketoconazole, ampicillin, iron salts).8,9 In addition, PPIs undergo metabolism by the hepatic isoenzymes, including CYP2C19 and CYP3A4. Clinically significant interactions are uncommon; however, a potentially important interaction exists between some PPIs and clopidogrel.8,9,11 Clopidogrel is a prodrug that requires activation by CYP2C19; therefore, PPIs have the potential to reduce its activation and subsequent antiplatelet effects.11 There is conflicting evidence about the clinical implication of this interaction; thus, it is recommended that a PPI with less CYP2C19 inhibition such as pantoprazole or rabeprazole be used.8,9,11
Histamine-2 Receptor Antagonists: H2RAs produce their effect by competing with histamine for binding to the H2 receptor on gastric parietal cells, leading to suppression of both the basal and stimulated acid secretion. In addition, other acid-promoting substances such as gastrin and acetylcholine have a reduced effect on parietal cells. H2RAs are also effective in treating nocturnal acid secretion, which is largely due to histamine.8,9
H2RAs are rapidly absorbed, inhibit 60% to 70% of daily acid production, and have a duration of action of approximately 10 hours. They undergo hepatic metabolism; however, in patients with renal dysfunction, dose adjustments are recommended. Common adverse events include fatigue, headache, diarrhea, myalgia, and constipation. Medically ill patients may have the potential to develop nosocomial pneumonia with H2RA use. Rarely, more serious adverse effects such as blood dyscrasias may occur.8,9
As with the PPIs, H2RAs raise the gastric pH and can subsequently inhibit the absorption of drugs requiring an acidic environment. In addition, cimetidine inhibits CYP1A2, CYP2C9, CYP2D6, and CYP3A4 and has the potential for many drug interactions.8,9
Sucralfate: This drug, used to prevent and treat ulcers, is a polymer of sucrose octasulfate bound to aluminum hydroxide. Within an acidic environment, sucralfate cross-linking occurs, forming a viscous paste that adheres to ulcers for up to 6 hours. It is postulated that once this agent forms a barrier over the ulcer, it can restrict further injury and block pepsin-mediated hydrolysis of mucosal proteins and stimulate prostaglandins and epidermal growth factor.8,9
Although sucralfate is not absorbed systemically and therefore has a low risk of adverse effects, constipation and aluminum toxicity may occur in patients with poor renal function.8,9 Since sucralfate is activated by acid, it should be administered on an empty stomach and separated from antacids by at least 30 minutes.9 It may also decrease the absorption of certain medications (e.g., digoxin, fluoroquinolones, phenytoin, ketoconazole), so special attention to potential drug interactions should be paid, especially since sucralfate is dosed four times per day and it may be challenging to separate these drugs.8,9
Clinical Studies
AEF is a rare condition; therefore, studies identifying therapies to prevent this complication are limited. GI protective agents discussed above have been considered for this off-label indication. Few studies have been conducted to study the effects of GI protective agents in this population. Most commonly, PPIs have been studied for the treatment of esophageal ulcers caused by AF ablations and for the prevention of the progression to AEF. Although H2RAs are also considered therapeutic alternatives to PPIs, studies investigating H2RAs for this indication have not been published. Sucralfate, however, has been studied as an adjunct to PPI therapy in one study. This section will include a discussion of these studies.
Animal Trials: The presentation of AEF is usually delayed; therefore, it is unlikely that it is caused by direct mechanical damage from the procedure.1 A study using a canine model was conducted to determine the factors associated with esophageal ulcer progression after ablation using ultrasound energy.7 An acute esophageal ulcer was present immediately after ablation in 18 out of the 20 dogs. The two dogs that did not develop ulcers had a maximum LET of 41°C, while the 18 dogs that developed ulceration had a maximum LET ³50°C. In 54% of the dogs, progression of the esophageal ulcer size (determined by follow-up endoscopy or autopsy) was associated with esophagitis and relaxation of the LES, suggesting the presence of gastroesophageal reflux. Mortality occurred in two of the dogs that had experienced progression of ulceration and developed a fever due to AEF.7
Human Trials: In a retrospective study of 425 patients who received left atrial RF catheter ablation, 77.2% (328/425) were noted to have macroscopic pathologic findings upon endoscopy 1 to 3 days post procedure.12 The most common findings included gastric/duodenal erosions and erythema, gastroparesis, reflux esophagitis, and hiatal hernia. None of these patients developed AEF, but it is important to note that it is common for patients to have GI complications after RF ablation.12
The following human trials involved prospective studies with PPIs, which are also summarized in TABLE 2.13-15
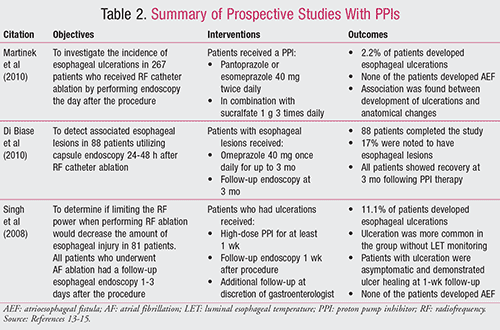
A prospective study was conducted to investigate the incidence of esophageal ulcerations in 267 patients who received RF catheter ablation.13 Endoscopy was performed in every patient the day after the procedure, and patients who had esophageal wall changes or injuries received more surveillance in addition to pharmacologic therapy, including a PPI (pantoprazole or esomeprazole 40 mg twice daily) in combination with sucralfate 1 g three times daily. This study found that 2.2% (6/267) of the patients developed esophageal ulcerations and none of these patients developed AEF. During the univariate analysis, the authors found there was an association between development of ulcerations and anatomical changes, such as left atrial enlargement, possibly due to sandwiching of the esophagus between the left atrium and thoracic spine. No other findings, in terms of patient tolerance or adverse effects, were reported.13
A smaller prospective study of 88 patients who underwent RF ablation was performed, also looking at esophageal complications.14 Patients received capsule endoscopy 24 to 48 hours post procedure. Seventeen percent of patients (15/88) were noted to have esophageal lesions. All of these patients then received oral PPI therapy with omeprazole 40 mg once daily. A follow-up endoscopy performed at 3 months showed recovery in all patients. No other findings, in terms of patient tolerance or adverse effects, were reported.14
A prospective observational study by Singh et al was conducted to determine if limiting the RF power to no more than 35 watts when performing the ablation would decrease the amount of esophageal injury.15 All patients who underwent AF ablation had a follow-up esophageal endoscopy 1 to 3 days after the procedure. Approximately 11% of the patients included in the study (9/81) had esophageal ulceration, and it was found to be more common in the group without LET monitoring. All patients who had ulcerations received a high-dose PPI (drug and dose not specified) for at least 1 week and had a follow-up endoscopy 1 week following the procedure to ensure ulcer healing. All patients with ulceration were asymptomatic and demonstrated ulcer healing at 1-week follow-up, and none of the patients developed AEF. No other findings, in terms of patient tolerance or adverse effects, were reported.15
Pharmacist’s Role
Pharmacists can play a key role in preventing the progression of esophageal ulcers to AEF following AF ablation. Prescribers may be routinely recommending PPI or H2RA therapy to patients for up to 4 weeks based on the 2012 Catheter and Surgical Ablation of Atrial Fibrillation guidelines.1 When communicating with these patients, it is important to weigh the benefits of PPIs and other therapies in preventing progression to AEF to the risks and complications associated with their use. Pharmacists should be aware of this common practice, and they can help to ensure that therapy is utilized for an appropriate time period following ablation.
Monitoring for any potential adverse events will also become increasingly significant to ensure proper compliance. PPIs and H2RAs raise the GI pH; therefore, pharmacists should screen for potential drug interactions and provide counseling to patients on appropriate timing of administration. Drug interactions can include metabolic inhibition of certain medications; therefore, dose adjustments may be necessary. If patients have concerns with administration of their medications, such as trouble swallowing from esophageal injury, recommendations about other dosage forms can be offered. Sucralfate, for example, is available in a liquid preparation and esomeprazole is available as granules that can be sprinkled over applesauce for easier swallowing (see also TABLE 1).8,9
Conclusion
It is routine practice that PPIs, H2RAs, and sucralfate are prescribed as off-label medications to prevent the development of AEF from esophageal ulcers post AF ablation. Based on the available studies, we agree that the possible benefit these medications offer to prevent this life-threatening condition may be warranted. However, this practice is based mostly on anecdotal experience and small studies. Patients using GI protective therapy after ablation did see some benefit in small studies. Although it is difficult to perform large-sample, prospective studies on a rare occurrence such as AEF, future studies are warranted to give more insight as to specific drug therapies, dosages, duration, and safety of using GI protective agents in AF ablation.
REFERENCES
1. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9(4):632-696.
2. Spragg DD, Tomaselli GF. Chapter 231. Principles of electrophysiology. In: Longo DL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
3. Cappato R, Calkins H, Chen SA, et al. Prevelance and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2009;53:1798-1803.
4. Lester M, Patel B. Atrial-esophageal fistula as a complication of cardiac radiofrequency catheter ablation [meeting abstract]. Chest. 2011;140(4):99A.
5. Maan A, Shaikh A, Mansour M, et al. Complications from catheter ablation of atrial fibrillation: a systematic review. Crit Pathways Cardiol. 2011;10(2):76-83.
6. Nakagawa H, Seres KA, Jackman WM. Limitations of esophageal temperature-monitoring to prevent esophageal injury during atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2008;1(3):150-152.
7. Yokoyama K, Nakagawa H, Seres KA, et al. Canine model of esophageal injury and atrial-esophageal fistula after applications of forward-firing high-intensity focused ultrasound and side-firing unfocused ultrasound in the left atrium and inside the pulmonary vein. Circ Arrhythm Electrophysiol. 2009:2(1):41-49.
8. McQuaid, Kenneth R. Chapter 62. Drugs used in the treatment of gastrointestinal diseases. In: Basic & Clinical Pharmacology. 12th ed. New York, NY: McGraw-Hill; 2012.
9. Wallace JL. Chapter 45. Pharmacotherapy of gastric acidity, peptic ulcers, and gastroesophageal reflux disease. In: Sharkey KA, ed. Goodman & Gilman’s the Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011.
10. Lexicomp Online [online database]. Lexi-Drugs. Hudson, Ohio: Lexi-Comp, Inc. www.lexi.com. Accessed September 1, 2014.
11. Simon T, Steg PG, Gilard M, et al. Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome P450 2C9 genotype in a large nationwide cohort of acute myocardial infarction: results from the French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) registry. Circulation. 2011;123:474-482.
12. Knopp H, Halm U, Lamberts R, et al. Incidental and ablation-induced findings during upper gastrointestinal endoscopy in patients after ablation of atrial fibrillation: a retrospective study of 425 patients. Heart Rhythm. 2014;11(4):574-578.
13. Martinek M, Meyer C, Hassanein S, et al. Identification of a high-risk population for esophageal injury during radiofrequency catheter ablation of atrial fibrillation: procedural and anatomical considerations. Heart Rhythm. 2010;7(9): 1224-1230.
14. Di Biase L, Dodig M, Saliba W, et al. Capsule endoscopy in examination of esophagus for lesions after radiofrequency catheter ablation: a potential tool to select patients with increased risk of complications. J Cardiovasc Electrophysiol. 2010;21(8): 839-844.
15. Singh SM, d’Avila A, Doshi SK, et al. Esophageal injury and temperature monitoring during atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2008;1(3):162-168.





