Multiple sclerosis (MS) was first described over 100 years ago, yet it remains unfamiliar to many clinicians.1 MS is a chronic neurologic disorder that affects the central nervous system (CNS).2 It is characterized by the impairment of nerve transmission down the axon, attributed to chronic unpredictable inflammation and demyelination of neurons.3 In other words, unusual lesions damage the protective coat (myelin) of nerve fibers, thus inhibiting the messages sent within the brain and to the spinal cord. Clinical presentation of MS is associated with exacerbations of weakness, imbalance/incoordination, spasticity, fatigue, bladder/bowel difficulties, vision loss, altered sensory perception, cognitive impairment, and neuropathic pain.4 Such signs and symptoms have a broad differential diagnosis, and therefore diagnosing MS is difficult when they initially present as an isolated clinical event. However, the clinical course over time--multifocal attacks, with an increasing burden of new demyelinative lesions seen on MRI scans and characteristic cerebrospinal fluid (CSF) findings--enable the diagnosis to be made.5 Furthermore, there is no single test for MS, and diagnosis is based on a history of attacks (i.e., at least one month apart) and myelin destruction in more than one area (i.e., brain and spinal cord).
The treatment of MS is limited to treating exacerbations, managing symptoms, improving function, and using disease-modifying drugs (DMDs). Treatment efforts are utilized in an attempt to improve the patient's quality of life. The cost of therapy can be substantial, since insurance companies may not cover the cost of DMDs without a firm diagnosis of clinically definite MS. The use of DMDs results in fewer new demyelinative lesions over time, but progressive neurologic disability is common, particularly when axonal degeneration occurs in response to chronic ongoing inflammation within the adjacent myelin sheath.
Epidemiology and Pathophysiology
According to the National Multiple Sclerosis Society, there are almost 400,000 diagnosed cases in the United States, yet the numbers may be higher due to patients having mild symptoms and seeking no treatment.6 Typically, MS begins early in adulthood, with the onset of symptoms at age 28 years and diagnosis at 33 years. Women are two times more likely to be diagnosed with MS. Caucasians have the highest incidence of MS, whereas persons of Japanese decent have the lowest. Geographical mapping has shown concentrated areas of increased risk for the development of MS. However, no concrete data have been shown as to why certain regions have a higher incidence of MS, although genetic susceptibility and ethnic migratory patterns are likely involved.
Although the pathogenesis of MS is still unknown, viral and autoimmune hypotheses lead the numerous theories as to its cause.7 These hypotheses include immune system malfunction, environmental factors, genetics, susceptible blood-brain barrier (BBB), diet, vitamin deficiencies, and allergic reactions.7 The common thread in all hypotheses is the resulting inflammatory response and sclerosis of the neuron. T-cells (CD4+ or CD8+) can produce inflammatory demyelination of the CNS,8 and myelin specific CD8+ T-cells are more abundant in patients with relapsing-remitting MS (RRMS).7 MS is also thought to be mediated by helper T-cells type 1 that produce interferon-gamma and other pro inflammatory cytokines.9
During a relapse, patients with MS experience an inflammatory response in which the myelin of the nerve cell gets stripped, otherwise known as demyelination.10,11 The inflammation also damages the axonal membrane, thus inhibiting the action potential along the nerve from the brain to the spinal cord. In addition, the inflammatory response damages the oligodendrocytes, which are the myelin-producing cells within the CNS. Researchers are now asking whether oligodendrocyte death causes inflammation, or if inflammation causes oligodendrocyte death. MS remission is characterized by a reduction in the inflammatory response and subsequent healing. The demyelinated axon increases sodium channels, which are integral in sending the action potential down the neuron. Since the CNS is a "plastic" organ, it can develop new neuronal pathways around the damaged neurons; however, this is not a speedy process. Oligodendrocytes sponsor remyelination, although axons do not function as well theoretically, and patients may not notice they have had a relapse. However, remyelination may not take place, resulting in a dysfunctional nerve, and the function of the underlying axon may become permanently lost. This lost myelin can be replaced with scar tissue, hence the name multiple (many) sclerosis (scar-forming).
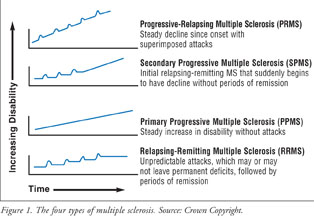
There are four types of MS: RRMS, secondary progressive MS (SPMS), primary progressive MS (PPMS), and progressive-relapsing MS (PRMS) (Figure 1).12 Approximately 80% of patients with MS have RRMS, which is characterized by episodes of symptoms (relapse) followed by the absence of symptoms (remission). Symptoms may evolve over days and disappear, although this varies between patients, and relapse appears approximately every two years. SPMS occurs as a result of RRMS developing into a progressive state. In this case, the patient previously would have been diagnosed with RRMS, but is now presenting with fewer relapses, with continual progression of neurologic symptoms. PPMS occurs when the patient's symptoms steadily increase proportional with time, and there are no episodes of relapse or remission. This form of MS has short periods where symptoms stabilize or improve slightly. The rarest form of MS is PRMS, which is expressed as gradual worsening with superimposed relapse/remission episodes.
Clinical Presentation and Diagnosis
MS can cause a variety of symptoms, including changes in sensation (hypesthesia), muscle weakness, spasticity, tremor, incoordination, ataxia, dysarthria, dysphagia, visual disturbances (e.g., nystagmus, optic neuritis, diplopia), fatigue, acute or chronic neuropathic pain (e.g., trigeminal neuralgia, dysesthesias), bladder/bowel difficulties, cognitive impairment, and affective lability.13-20 The initial attack of MS is generally mild and self-limiting, and it is usually identified in retrospect once the diagnosis of MS is made. The wide array of signs and symptoms of MS is due to the variability of lesions within the CNS white matter. A patient may present with hypesthesia and diplopia during one relapse and tremors the next. As the demyelinative plaque burden increases, cognitive manifestations may appear. Depending on the type of MS, symptoms may result in progressive neurologic disability.
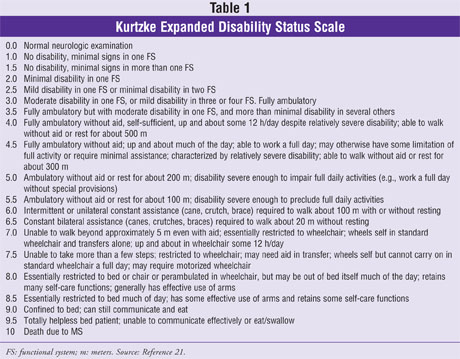
Patients are monitored over time with a meticulous neurologic exam, MRIs, and the use of the Kurtzke Expanded Disability Status Scale (EDSS).21,22 Serial lumbar punctures (LPs) are not necessary once the diagnosis has been established. The neurologic exam assesses changes in motor/sensory function, reflexes, coordination, gait, and cranial nerves. Common abnormal signs include the Babinski reflex (a positive test in the patient has an upward curling of the toes), and the Romberg test (which examines proprioception from the lower extremities).
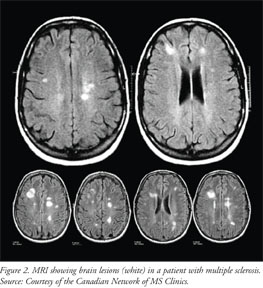
The most sensitive test to detect lesions and changes in the CNS of patients with MS is the MRI.22 Neurologists use serial MRIs to confirm diagnosis and monitor worsening of symptoms. Gadolinium enhancement is used to detect a compromised BBB or lesions with active inflammation (Figure 2).4 Once axonal loss occurs, dark zones (black holes) may appear within the plaques.
A LP or a spinal tap is used to rule out other diseases and also help confirm the MS diagnosis and prognosis.23 The CSF typically shows an increase in IgG synthesis, oligoclonal banding, and elevation of myelin basic proteins. The EDSS measures the progression of MS using a rating scale between 0 and 10 (Table 1). A patient with a normal neurologic exam will rate a 0, whereas a patient who is unable to walk beyond five yards without aid and is mostly confined to a wheelchair would rate a 7.0 on the scale.21
Treatment of MS
Since there is currently no cure for MS, treatment is limited to treating exacerbations, managing symptoms, improving function, and using DMDs.4,14-20 Treating exacerbations or inflammation in the CNS is limited to a short course of corticosteroids (e.g., prednisone, prednisolone, methylprednisolone, betamethasone, or dexamethasone) to reduce inflammation.4 Short-term side effects of steroids are increased appetite, psychosis, bloating, acne, insomnia, headache, and muscle weakness. Due to the long-term side effects of bone loss, suppression of the immune system, moon face, blood glucose changes, and stomach ulcers, this treatment is limited to short courses of therapy.
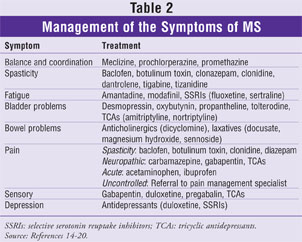
The management of symptoms is the next level of treatment in patients with MS. The treatment options used for some of the symptoms exhibited by patients are listed in Table 2.
Rehabilitation is the primary method of improving function in patients with MS. Depending on the level of disability, patients may require physical, occupational, speech, or even cognitive therapy. For long-term treatment of MS, most physicians will prescribe DMDs to their patients.
Disease-Modifying Drugs
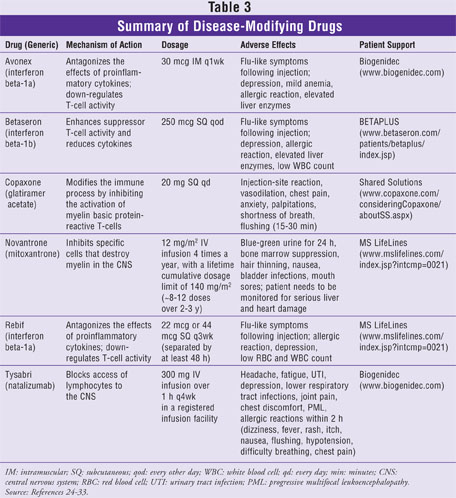
DMDs have been shown to reduce the frequency and severity of clinical attacks in patients with MS.24-33 These drugs are taken on a long-term basis and have been shown to be the best defense in slowing down the natural course of MS. DMDs will not prevent recurring symptoms, and they are not approved to treat PPMS. The National Multiple Sclerosis Society's medical advisory board agrees that DMDs are most effective when started early in the course of the disease. The FDA has approved six DMDs for the treatment of MS: Avonex (interferon beta-1a), Betaseron (interferon beta-1b), Copaxone (glatiramer acetate), Rebif (interferon beta-1a), Novantrone (mitoxantrone), and Tysabri (natalizumab). Avonex is reserved for the relapsing forms of MS and a single episode of MS if MRI features are consistent. Betaseron is approved to treat relapsing forms of MS and SPMS with relapses. Copaxone and Rebif are reserved for the treatment of RRMS only. Finally, Novantrone is used to treat worsening RRMS and is indicated for PRMS or SPMS, while Tysabri treats relapsing forms of MS as monotherapy. The mechanisms of action, dosages, and adverse effects of DMDs are illustrated in Table 3.
DMDs are designed to reduce the occurrence of new demyelinating lesions, resulting in reduced frequency and severity of attacks, and to prevent permanent damage to nerve axons.24-33 The advantages and disadvantages of each DMD are related to adverse effects, dosing schedules, routes of administration, and expense. Generally, these drugs are very costly and are not covered under most formulary plans; however, since they are injectable, some insurance plans will provide reimbursement under the medical benefit instead of under the drug benefit. In addition, each DMD has an industry-sponsored association that provides support, financial assistance, and patient information. For example, if patients are on Copaxone, they would be supported by the program Shared Solutions (Table 3).
Conclusion
MS has long been known, yet diagnosis remains difficult due to the complexity of the disease and its wide array of signs and symptoms. Treating MS still relies on symptomatic relief, but therapeutic advances in the form of DMDs have shown promising results. Although research in MS has been evolving rapidly, there still remains no cure for the disease. Pharmacists can play a vital role by understanding the complexity of MS and being able to rule out differential diagnosis when patients present to the practice setting. As well as being drug experts, pharmacists should be able to counsel patients on DMDs, as these medications are quite complicated and overwhelming to many patients.
ACKNOWLEDGEMENT: The authors wish to thank Michael Watters, MD, Professor of Medicine, Division of Neurology, at the University of Hawaii John A. Burns School of Medicine, for providing selected references and critical review of this manuscript.
REFERENCES
1. Compston A, Ebers G, Lassmann H, et al. McAlpine's Multiple Sclerosis. 3rd ed. London, UK: Churchill Livingstone; 1998.
2. Ringold, S, Lynm C, Glass RM. JAMA patient page. Multiple sclerosis. JAMA. 2006;296:2880.
3. Frohman E, Racke MK, Raine C. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med.
4. Noseworthy J, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938-952.
5. Freedman MS, Thompson EJ, Deisenhammer F, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol. 2005;62:865-870.
6. Sadovnick AD, Dyment D, Ebers GC. Genetic epidemiology of multiple sclerosis. Epidemiol Rev.
7. Lucchinetti CF, Rodriquez M. The controversy surrounding the pathogenesis of the multiple sclerosis lesion. Mayo Clin Proc. 1997;72:665-668.
8. Huseby ES, Liggitt D, Brabb T, et al. A pathogenic role for myelin-specific CD8+ T cells in a model for multiple sclerosis. J Exp Med. 2001;194:669-676.
9. Zang YC, Li S, Rivera VM, et al. Increased CD8+ cytotoxic T cell responses to myelin basic protein in multiple sclerosis. J Immunol. 2004;172:5120-5127.
10. Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain. 2002;125:338-349.
11. Lucchinetti C, Bruck W, Parisi J, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707-717.
12. Andersson PB, Waubant E, Gee L, Goodkin DE. Multiple sclerosis that is progressive from the time of onset: clinical characteristics and progression of disability. Arch Neurol. 1999;56:1138-1142.
13. Crayton H, Heyman RA, Rossman HS. A multimodal approach to managing the symptoms of multiple sclerosis. Neurology. 2004;63(suppl 5):S12-S18.
14. Kumar D. Approved and investigational uses of modafinil: an evidence-based review. Drugs.
15. Chou R, Peterson K, Helfand M. Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systematic review. J Pain Symptom Manage. 2004;28:140-175.
16. Kay GG, Ebinger U. Preserving cognitive function for patients with overactive bladder: evidence for a differential effect with darifenacin. Int J Clin Pract. 2008 Aug 11. Epub.
17. Finnerup NB. A review of central neuropathic pain states. Curr Opin Anaesthesiol.
18. Svendsen KB, Jensen TS, Overvad K, et al. Pain in patients with multiple sclerosis: a population-based study. Arch Neurol. 2003;60:1089-1094.
19. Dworkin RH, Backonja M, Rowbotham MC, et al. Advances in neuropathic pain: diagnosis, mechanism, and treatment recommendations. Arch Neurol. 2003;60:1524-1534.
20. Daly E, Komaroff AL, Bloomingdale K, et al. Neuropsychological function in patients with chronic fatigue syndrome, multiple sclerosis, and depression. Appl Neuropsychol. 2001;8:12-22.
21. Kurtzke JF. Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444-1452.
22. Miller DH, Grossman RI, Reingold SC, McFarland HF. The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain. 1998;121:3-24.
23. Rinker JR III, Trinkaus K, Cross AH. Elevated CSF free kappa light chains correlate with disability prognosis in multiple sclerosis. Neurology. 2006;67:1288-1290.
24. Manfredonia F, Pasquali L, Dardano A, et al. Review of the clinical evidence for interferon beta 1a (Rebif) in the treatment of multiple sclerosis. Neuropsychiatr Dis Treat. 2008;4:321-336.
25. Goodin D. Comparative studies of glatiramer acetate and interferon beta. Int MS J. 2008;15:39-41.
26. Moses H Jr, Brandes D. Managing adverse effects of disease-modifying agents used for treatment of multiple sclerosis. Curr Med Res Opin. 2008;24:2679-2690.
27. IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655-661.
28. Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol. 1996;39:285-294.
29. Fox EJ. Management of worsening multiple sclerosis with mitoxantrone: a review. Clin Ther. 2006;28:461-474.
30. Scott LJ, Figgitt DP. Mitoxantrone: a review of its use in multiple sclerosis. CNS Drugs.
31. Novak J, Lovett-Racke AE, Racke MK. Monoclonal antibody therapy and neurologic disorders. Arch Neurol. 2008;65:1162-1165.
32. Brod SA, Marshall GD Jr, Henninger EM, et al. Interferon-beta 1b treatment decreases tumor necrosis factor-alpha and increases interleukin-6 production in multiple sclerosis. Neurology.
33. Dhib-Jalbut S. Mechanism of action of interferons and glatiramer acetate in multiple sclerosis. Neurology. 2002;58(suppl 4):S3-S9. 2006;354:942-955. 1997;19:99-106. 2008;68:1803-1839. 2008;21:586-589. 2004;18:379-396. 1996;46:1633-1638.






