US Pharm. 2009;34(7):HS2-HS8.
Tuberculosis (TB) is a widespread disease found in both poorly developed and well-developed countries. TB can be a devastating and deadly disease despite advances in therapy. It primarily infects the lungs and is classified as either latent or active. Latent tuberculosis is treated in order to avoid progression to active tuberculosis disease.
Epidemiology
There were over 9 million new cases of TB and 1.7 million deaths worldwide from the disease in 2006.1 The vast majority of the deaths are in poorly developed countries. Fortunately, TB is both preventable and curable. However, according to the World Health Organization, nearly four out of 10 cases of TB are not being properly detected and treated.1
In 2004, there were 14,502 new cases of TB and 657 deaths in the United States. Since 1993, the number of new cases has fallen annually, reaching a new record low of 12,898 (4.2 per 100,000) in 2008.2,3 Although the U.S. rate is low, foreign-born individuals have a rate 10 times higher than those born in the U.S., with Hispanics, Africans, and Asians having a rate 8, 8, and 23 times higher, respectively, than that of non-Hispanic whites.3 Age is also a risk factor for TB. In 2006, the highest rate was seen in adults over the age of 65 years (7.2 per 100,000), while the lowest rate was in children from birth to 14 years (1.3 per 100,000).2
Etiology
Mycobacterium tuberculosis is the causative organism. It is spread person to person through the air by infectious droplets produced during coughing, sneezing, or speaking. Exposure to TB does not guarantee that a person will become infected. Upon inhalation, the infectious droplets enter the lungs and reach the alveoli where the organisms replicate. Macrophages (white blood cells that help initiate defense mechanisms) then engulf the mycobacteria, followed by the formation of a granuloma (collection of immune cells) that contains the bacteria. If the organisms remain contained in the granuloma, the person has latent TB and cannot transmit the organisms to others at that time.
In active TB, the host is unable to contain the organisms. Macrophages that had previously contained the organisms rupture and release them into the bloodstream. At this point, the organisms may infect the lungs, which is most common, but they may also infect other areas (extrapulmonary TB).4 Examples of other sites include the peritoneum, intestines, or pleural cavity. Lymph nodes, bones, joints, and kidneys may also be involved. Extrapulmonary sites may be more common among children and patients with impaired immunity.5
Approximately 10% of people infected with M tuberculosis will progress from latent TB to active TB if not treated.4 The risk of progressing to active TB is higher in the first 2 years following initial infection. Factors that determine progression from latent TB to active TB include the number and virulence of organisms inhaled and the host’s cell-mediated immune response.4 Patients who have a history of IV drug use, are underweight, or have medical conditions such as HIV/AIDS or diabetes are at increased risk of progression from latent to active TB.6
Diagnosis and Classification
Evaluation of TB includes a patient history and physical to obtain the history of TB exposure, travel history, presenting signs and symptoms, and general health information.4 Latent TB generally does not produce any signs or symptoms. In active TB, signs and symptoms typically include a persistent, productive cough, fever, and night sweats. Individuals may also experience weight loss and fatigue. A person with suspected active TB may have a chest x-ray to identify infiltrates or consolidations in the lungs. Microbiological studies performed on clinical specimens, such as sputum analysis, are also used for diagnosis. The detection of acid-fast bacilli in stained smears examined microscopically provides preliminary confirmation of TB.4 Definitive diagnosis of active TB involves culturing M tuberculosis.6
The tuberculin skin test is the standard for identifying latent TB. Purified protein derivative (PPD) tuberculin is isolated from culture filtrate and is used for most skin testing. An individual infected with latent TB will produce a delayed-type hypersensitivity reaction to an intradermal injection of PPD tuberculin. The reaction begins 5 to 6 hours after the injection, peaks at 48 to 72 hours, and then subsides over a few days.6 Several factors may affect the results of the tuberculin skin test. Many parts of the world use the bacillus Calmette-Guérin (BCG) vaccine to prevent TB, although its efficacy is uncertain. A person who has been vaccinated with BCG and is not infected with TB will also react to the tuberculin skin test. If a skin test is positive, further examination is necessary to determine if the person has active or latent TB.4
The CDC and American Thoracic Society recommend targeted tuberculin testing for latent TB. This is a strategy that attempts to identify persons at high risk for developing active TB who would benefit from treatment. High-risk individuals include persons on immunosuppressant drugs, those who have come in contact with a person who has infectious TB, those who are HIV positive, IV drug users, and immigrants who have come from high-prevalence countries within the past 5 years. This list is not exhaustive. Infected persons considered high risk should be offered treatment of latent TB infection regardless of age.5,6

Treatment
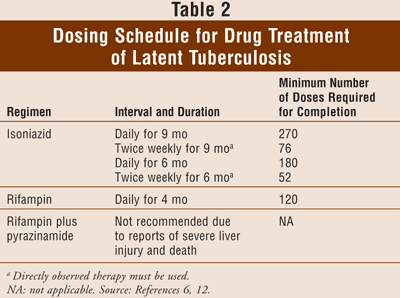
Treatment of latent TB typically consists of isoniazid 300 mg daily for 9 months (TABLES 1 and 2). Six months of daily isoniazid treatment also provides substantial protection and may be a cost-effective alternative to 9 months in some cases. An alternative treatment is rifampin daily for 4 months. The choice of the regimen to treat latent TB is based on length and complexity of the regimen, potential drug interactions and side effects, and drug resistance, which can be a problem.6
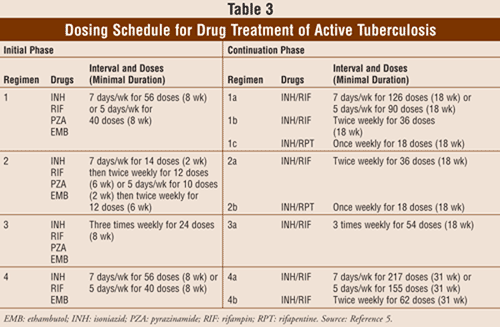
Treatment of active TB lasts for either 6 or 9 months and requires an initial 2-month phase followed by a continuation phase of either 4 or 7 months. Patients receiving the 6-month regimen will take four medications in the initial phase. Choice of treatment for active TB is based on isoniazid resistance (TABLE 3). Ethambutol is not necessary in the initial phase if testing shows isoniazid and rifampin susceptibility.5 The continuation phase includes isoniazid and rifampin given daily, twice weekly, or 3 times weekly for a minimum of 4 months. Patients receiving the 9-month regimen for active TB follow a three-drug regimen during the initial phase. The continuation phase includes isoniazid and rifampin given daily or twice weekly. Alternative regimens include the following: rifampin, pyrazinamide, and ethambutol for 6 months; rifampin and ethambutol for 12 months with pyrazinamide for the first 2 months; isoniazid, ethambutol, and a fluoroquinolone for 12 to 18 months with pyrazinamide for the first 2 months. Contacts of patients with active TB should be referred for evaluation and will typically have a tuberculin skin test. Contacts with positive skin tests, and even some contacts with negative skin tests, are generally treated for TB.5
Isoniazid: Isoniazid is bactericidal against M tuberculosis and is widely used for treatment of TB. Among adherent participants in clinical trials, it has a protective efficacy of approximately 90%.6 Isoniazid may cause liver damage associated with increased liver function tests (LFTs). Liver enzymes may increase up to five times the upper limit of normal in 10% to 20% of patients receiving isoniazid for latent TB, but the levels usually return to normal with continued administration of isoniazid.7 Approximately 1% of patients will experience clinical hepatotoxicity due to isoniazid.8 Patients taking isoniazid should avoid alcohol in order to prevent hepatotoxicity. However, acetaminophen administration at therapeutic doses combined with isoniazid is considered safe.9 Isoniazid interferes with the metabolism of pyridoxine and may cause dose-related peripheral neuropathy or neurotoxicity due to pyridoxine deficiency. Patients may take pyridoxine 25 mg daily to prevent this uncommon side effect if they are pregnant, breast-feeding, or have conditions associated with neuropathy such as diabetes, uremia, malnutrition, or HIV infection.6 Routine monitoring for isoniazid including LFTs is not necessary, but may be performed monthly if symptoms of hepatotoxicity occur or if a patient develops abnormal liver function that does not require discontinuation.5 Symptoms of hepatotoxicity include nausea, vomiting, abdominal pain, and loss of appetite. Isoniazid is a relatively potent inhibitor of several CYP450 enzymes and may increase the risk of toxicity of certain drugs such as anticonvulsants (e.g., phenytoin, carbamazepine) and some benzodiazepines (e.g., diazepam, triazolam).5
Rifampin: Rifampin is a first-line rifamycin derivative and bactericidal medication used to treat TB. Side effects include gastrointestinal (GI) upset, which is rarely severe enough for discontinuation, and cutaneous reactions. Rifampin causes an orange discoloration of body fluids such as urine, tears, and sweat. Clothing or soft contact lenses may be permanently discolored as a result. Hepatitis is possible with rifampin, although this is more common when the drug is given concurrently with isoniazid. Rifampin induces hepatic enzymes and has several clinically significant interactions with other drugs, including oral contraceptives, protease inhibitors, and non-nucleoside reverse transcriptase inhibitors.5 Routine laboratory monitoring tests are not required for rifampin. However, the presence of drug interactions may necessitate regular measurements of blood levels of interacting drugs such as the anticonvulsants and benzodiazepines.5,6
Rifabutin: Rifabutin is a second-line rifamycin derivative with a longer half-life and fewer drug interactions than rifampin. Rifabutin interacts with protease inhibitors, although they can be used together. Most M tuberculosis strains that are resistant to rifampin are also resistant to rifabutin. Hepatotoxicity with rifabutin is rare (<1%). Like rifampin, rifabutin may cause orange discoloration of body fluids. Rifabutin may also cause dose-related arthralgias and/or myalgias. Other side effects include neutropenia, rash, and GI upset. Liver function monitoring for rifabutin is not necessary.5
Rifapentine: Rifapentine is a rifamycin derivative with a long half-life. It is administered once a week with isoniazid in the continuation phase of treatment in patients with negative sputum smears at completion of the initial phase of treatment. Adverse effects and monitoring are similar to those for rifampin. Rifapentine may cause skin rash, hepatitis, and GI upset. As with rifampin, it may also cause orange discoloration of body fluids. It should not be used for HIV-positive patients due to development of resistant organisms and interactions with protease inhibitors.5
Pyrazinamide: Pyrazinamide is a bactericidal agent in an acidic environment and is consequently active against M tuberculosis inside of macrophages. Side effects include GI upset, mild nausea, and hepatotoxicity. Hyperuricemia may occur but rarely causes acute gout. However, serum uric acid is a surrogate marker for adherence with this medication regimen. Polyarthralgias occur in up to 40% of patients receiving pyrazinamide, but rarely require dose adjustment or drug discontinuation.5 Pyrazinamide does not interact with antiretroviral medications. LFTs may be monitored in a patient with underlying liver disease.5
Ethambutol: Ethambutol is included in initial treatment of active TB to prevent rifampin resistance when isoniazid resistance is already present. Ethambutol may cause optic neuritis and is not recommended for children whose visual acuity cannot be monitored. Peripheral neuritis and cutaneous reactions are rare. Ethambutol is cleared primarily by the kidneys and requires dose adjustments when creatinine clearance is less than 70 mL/min.5 Monitoring includes baseline visual acuity testing, color discrimination testing, and monthly interviews to assess visual disturbances.5
Fluoroquinolones: Although not approved by the FDA to treat TB, the antibiotics levofloxacin, moxi floxacin, and gatifloxacin have been used for the treatment of active TB. The exact role of fluoroquinolones in treating active TB has not yet been established. These medications have been used to treat drug-resistant TB or patients who cannot take first-line drugs due to side effects.5 Use in children is limited because of possible effects on bone and cartilage growth. Other side effects include GI, neurologic, and cutaneous reactions.
Diagnostic and Treatment Advances
Interferon gamma (IFN-gamma) assays are currently available as diagnostic tests that measure the release of IFN-gamma in response to stimulation by M tuberculosis. The antigens used to stimulate response are more specific for M tuberculosis and are less likely than the tuberculin skin tests to cross-react with BCG vaccination. IFN-gamma has a potential role in detecting latent TB.10 Nucleic acid amplification (NAA) tests are currently available as an adjunct to conventional diagnostic techniques for active TB. The advantage of NAA testing is earlier laboratory confirmation of TB, leading to earlier treatment initiation. These tests amplify target nucleic acid regions that uniquely identify M tuberculosis. Guidelines provided by the CDC suggest that NAA testing should be performed on sputum from patients with signs and symptoms of pulmonary TB.11
Alternative medications or medication delivery systems may affect the future treatment of TB. Medications that may be evaluated for treatment of TB include those related to metronidazole (e.g., nitroimidazopyrans) and oxazolidinones such as linezolid. Alternative drug delivery systems include the use of liposomal encapsulation to direct medications to the site of infection. Medications delivered as inhalable microparticles may reduce dose requirements and toxicity while delivering the medication to the site of infection.5 Immunotherapy involving the administration of protective cytokines may prove to be adjuncts for the treatment of multidrug resistant TB.6
Pharmacist Involvement
Patient adherence to treatment regimens for TB is a significant problem. According to the CDC, only 60% of people who start treatment for latent TB complete at least 6 months of treatment.5 Consequences of nonadherence include possible treatment failure, relapse, and the emergence of drug-resistant strains of M tuberculosis.6 Initial testing, evaluation, and treatment can be very complex for patients. Factors such as length and complexity of regimens and medication side effects contribute to nonadherence. Pharmacists may play a role in treating TB by providing education to patients with latent TB in order in increase adherence. This is particularly important because these patients do not have clinical symptoms and may not be motivated to continue treatment. Pharmacists can also contribute to patient care by monitoring for side effects and drug interactions. For example, there are clinically significant drug interactions between certain TB medications and nonprescription medications such as antacids (TABLE 1).
The use of directly observed therapy (DOT) is an option for increasing adherence to TB treatment. For DOT, a pharmacist or other health care professional observes the patient ingesting each dose of medication. This is widely used for regimens of two to three doses in one week, but is generally not necessary with daily therapy. It is also the preferred initial strategy for children, those experiencing treatment failure, and patients in institutional settings.5
Conclusion
Treatment of TB can be difficult, requiring long courses of many medications to treat active disease. Treatment of latent TB is uniquely complex because patients lack signs and symptoms of the disease but are treated with long courses of therapy. Adherence to treatment is essential for avoiding drug-resistant TB and treatment failure. Providers who treat TB must strive for successful treatment of the disease as a matter of public health. Pharmacists who are involved in the treatment of TB are in an ideal position to encourage medication adherence.
REFERENCES
1. World Health Organization. 2008 tuberculosis facts. www.who.int/tb. Accessed March 31, 2009.
2. CDC. Reported Tuberculosis in the United States, 2006. Atlanta, GA: U.S. Department of Health and Human Services, CDC; September 2007.
3. CDC. Trends in tuberculosis—United States, 2008. MMWR. 2009;58:249-253.
4. American Thoracic Society. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376-1395.
5. CDC. Treatment of tuberculosis. MMWR. 2003;52:1-77.
6. American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161:S221-S247.
7. Mitchell JR, Zimmerman HJ, Ishak KG, et al. Isoniazid liver injury: clinical spectrum, pathology and probable pathogenesis. Ann Intern Med. 1976;84:181-192.
8. Kopanoff DE, Snider DE, Caras GJ. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis. 1978;117:991-1001.
9. Millard PS, Wilcosky TC, Reade-Christopher SJ, Weber DJ. Isoniazid-related fatal hepatitis. West J Med. 1996;164:486-491.
10. Nahid P, Pai M, Hopewell PC. Advances in the diagnosis and treatment of tuberculosis. Proc Am Thorac Soc. 2006;3:103-110.
11. CDC. Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR. 2009;58:7-10.
12. CDC. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for the treatment of latent tuberculosis infection—United States, 2003. MMWR. 2003;52:735-739.
To comment on this article, contact rdavidson@jobson.com.






