US Pharm. 2006;10:47-54.
Fluoroquinolones evolved from nalidixic acid, which was approved by the FDA in 1963. Nalidixic acid was reasonably effective against many gram-negative organisms; however, it did not possess adequate activity against many gram-positive, anaerobic, or other important gram-negative organisms, such as Pseudomonas aeruginosa.1,2 This narrow spectrum of activity limited the use of nalidixic acid to the extent that it was indicated only for the use of urinary tract infections (UTIs).
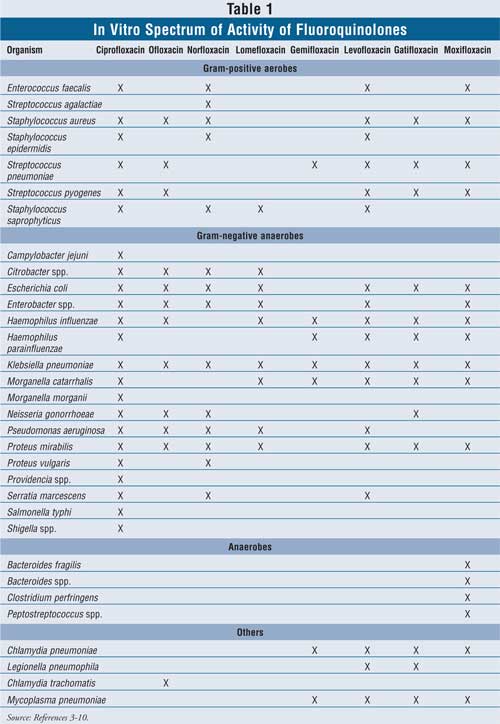
After the introduction of nalidixic acid, the addition of a fluoride atom was found to yield quinolones with a greater spectrum of activity, thus leading to "fluoroquinolones." Second-generation fluoroquinolones possessed improved activity against gram-negative organisms but still had limited activity against gram-positive organisms. With the release of the third- and fourth-generation fluoroquinolones, greater activity against gram-positive organisms, particularly Streptococcus pneumoniae, was gained, but activity against P. aeruginosa subsequently decreased. 3-10 The spectrum of in vitro activity for available fluoroquinolones is listed in TABLE 1.
Mechanism of Action
Fluoroquinolones are bactericidal
anti-infectives that work by inhibiting DNA synthesis through the formation of
a complex with either DNA gyrase or topoisomerase IV--the enzymes responsible
for removing coils in the DNA and separating the daughter strands. This
three-way complex between the DNA molecule, fluoroquinolone, and
gyrase/topoisomerase IV prevents DNA replication from occurring at the
replication fork, leading to cell death.11
Current fluoroquinolones may have common, mild side effects (e.g., nausea, dizziness, and headache) or other more serious side effects (e.g., phototoxicity, hepatotoxicity, QT prolongation, and tendinitis) and are not recommended for children. Yet, as a class, the fluoroquinolones are generally well tolerated.
Rates of Fluoroquinolone Prescribing
Over the years, the
susceptibility of microorganisms has been changing, possibly due to the misuse
of antimicrobial agents. Misuse not only refers to incorrect use of a drug for
a certain indication or microorganism but also applies to inappropriate
treatment duration and/or the use of doses that are too low, which may result
in the selection of mutant isolates.
Widespread use of fluoroquinolones may also contribute to the developing resistance of microorganisms. According to data published in the American Journal of Medicine, fluoroquinolones became the most frequently prescribed antibiotics in the United States between 1995 and 2002. During this time, there was a threefold increase in fluoroquinolone use: Prescriptions rose from seven million in 1995 to 22 million in 2002. This is a dramatic increase, particularly since the percentage of overall antibiotic prescriptions did not vary significantly during this period (12% in 1995; 11% in 2002). In addition to the increase in number of fluoroquinolone prescriptions, it was also found that 42% of these prescriptions were for non–FDA-approved indications.12
Based on research from a study conducted in Belgium, the rate of fluoroquinolone use nearly doubled from 1993 to 2003 with the introduction of levofloxacin and moxifloxacin. The majority of the fluoroquinolone consumption was used to treat UTIs, followed by lower respiratory infections, and to a lesser degree, upper respiratory tract infections.13
The information gained from these studies is fundamental in determining the impact of widespread use of fluoroquinolones, especially since evidence indicates there is a relationship between increasing microorganism resistance and the rising use of antibiotics. Thus, prudent use of fluoroquinolones is crucial, because resistance may increase with broad misuse of this class of agents.14-16
Mechanisms of Fluoroquinolone Resistance
Resistance to fluoroquinolones
can occur through several mechanisms. First, alterations can occur in the
target enzymes, to the extent that fluoroquinolones are unable to bind DNA
gyrase or topoisomerase IV. Second, alterations can occur in the permeability
of the cell, making it difficult for fluoroquinolones to enter and access the
target enzymes. Third, chromosomal mutations may occur that could affect the
affinity, or confer resistance, of the target enzymes for fluoroquinolones. In
addition, multidrug resistance (MDR) efflux pumps found in the membranes of
some bacteria may also confer resistance to fluoroquinolones.17,18
In China, there have been reports of resistance to quinolones in
Escherichia coli introduced through plasmid-mediated transfers.19
Emerging Resistance
Several studies have been
published regarding the evolving resistance of certain organisms to
fluoroquinolones. Of particular importance is the growing resistance of S.
pneumoniae. The CDC has already reported the organism's resistance to the
newest fluoroquinolone antimicrobials. S. pneumoniae is one of the most
common respiratory pathogens found in lower respiratory tract infections, such
as acute bronchitis, acute exacerbations of chronic obstructive pulmonary
disease, and pneumonia.20 Because treatment of lower respiratory
tract infections is based on empirical therapy, and due to their excellent
coverage of S. pneumoniae, the newer fluoroquinolones--levofloxacin,
moxifloxacin, gatifloxacin, and gemifloxacin (often termed the respiratory
fluoroquinolones)--have been pushed to the forefront in infections
involving this organism. This may add to the growing resistance seen in this
class of antibiotics. The mechanisms of resistance for S. pneumoniae
are thought to be due to chromosomal mutations and/or efflux pumps. Although
S. pneumoniae resistance to fluoroquinolones is currently low, news of
clinical failures and increasing resistance have already been reported.
18,21,22
Haemophilus influenzae is another important pathogen involved in respiratory tract infections, particularly pneumonia. Currently, fluoroquinolone resistance rates for this organism are low but not unheard of.23 Notably, one case report involves a 71-year-old woman with community-acquired pneumonia who died secondary to an infection involving H. influenzae, which was resistant to levofloxacin, ciprofloxacin, moxifloxacin, and gatifloxacin. The mechanism of resistance observed in this organism was due to mutations in the target enzymes.24
UTIs are very common and can be found in both the inpatient and outpatient settings. Some of the most typical urinary pathogens associated with UTIs are E. coli, Proteus mirabilis, Klebsiella pneumoniae, and the Enterobacter species. However, over the years, the susceptibility of these pathogens has been changing. Trimethoprim-sulfamethoxazole has been the mainstay of therapy for UTIs, but fluoroquinolones have now become first-line empirical therapy in some regions. This change may be a contributing factor to the growing resistance of these organisms to fluoroquinolones.25
In one study that evaluated the rising resistance rates of E. coli to fluoroquinolones in UTIs, resistance to ciprofloxacin and ofloxacin rose from 4.1% and 5.2% in 1996, to 25.3% and 27.6% in 2002, respectively.16 Additionally, another study that was conducted over a 12-year period found comparable increasing fluoroquinolone resistance rates in both the inpatient and outpatient settings, not only for E. coli but for P. mirabilis and Enterobacter cloacae as well. Similar increasing inpatient resistance rates were also reported for K. pneumoniae.15
Combating Antimicrobial Resistance
Resistance is on the rise even in
organisms with low-level resistance to fluoroquinolones. Fluoroquinolones are
an important class of antibiotics, and if resistance to these antimicrobials
becomes problematic, treatment options for certain infectious diseases will be
limited. In addition, resistance leads to the need for new antimicrobials;
however, the number of new antimicrobial agents produced over the years has
decreased. Research has shown that FDA approvals of antibacterial drugs have
declined by 56% from 1998 to 2002, compared to the period between 1983 and
1987. Of the 225 new molecular entities approved from 1998 to 2002, only seven
of those agents were antimicrobials. Furthermore, even though development of
new antibiotics within an existing class is advantageous, because it can lead
to enhancement of the spectrum of activity and safety data, new classes of
antimicrobials with differing mechanisms of action are imperative to combat
the increasing rise of multidrug-resistant organisms.14
Recently, the CDC, FDA, and National Institutes of Health teamed up with other key health organizations to form the Interagency Task Force on Antimicrobial Resistance, which is dedicated to creating a public health action plan to combat antimicrobial resistance.20 This plan comprises four areas of focus: surveillance, prevention and control, research, and product development. These four areas form the "blueprint" for measures that can be undertaken to address rising antimicrobial resistance.
Surveillance: According to the Task Force, "unless antimicrobial problems are detected as they emerge--and actions are taken quickly to contain them--the world may soon be faced with previously treatable diseases that have again become untreatable, as in the preantibiotic era."20 Thus, the Task Force considers it necessary to monitor patterns of antimicrobial use, as well as to enhance the current beliefs of the link between antimicrobial use and resistance.
Prevention and Control:The Task Force defines effective antimicrobial use as "use that maximizes therapeutic impact while minimizing toxicity and the development of resistance, and it is overuse and misuse that must be decreased to reduce the selective pressure favoring the spread of resistance."20 As stated earlier, effective use entails using the correct drug for the specific indication and microorganism, using the appropriate treatment duration and dosage, and using antimicrobial therapy only when it is beneficial to the patient. The Task Force concludes that strategies to endorse appropriate use involve informing health care providers and consumers about the recommendations and limitations surrounding antimicrobial agents, improving diagnostic techniques, carrying out a public health educational crusade, and employing educational and behavioral interventions that will aid health care providers in choosing the correct antimicrobial therapy for each patient.
Research: The basis for each of the focus points listed is research. There are three concentrated areas: identifying gaps and needs in the understanding of resistance, supporting a vigorous research community, and producing new antimicrobials from results of research.
Product Development:As indicated previously, antimicrobial approvals have decreased over the years, which is another concern of the Task Force. They recognize that the reduced rate of new antimicrobial approvals is not sufficient to address the escalating rate of microbial resistance. They attribute the lower rate to reluctant pharmaceutical companies, scientific limitations, and lack of awareness of the need for new antimicrobial agents. Thus, the focus of product development is to address these deficits by recognizing and publicizing the need for new agents and providing incentives to encourage the development of new antimicrobials.
Conclusion
Fluoroquinolone resistance is an
increasing problem not only in the U.S. but also worldwide, potentially due to
the widespread misuse of this class of antimicrobials. The Interagency Task
Force on Antimicrobial Resistance suggests that enhanced surveillance of
antimicrobial resistance is critical to extend the therapeutic use of
antimicrobials, including fluoroquinolones. In addition, the production of new
agents is vital to combat this mounting rate of resistance. However, until new
agents can be produced, stricter guidelines should be initiated to preserve
this broad class of antibiotics and others as well. Furthermore, the public
and health care providers should be educated on the impact of inappropriate
antimicrobial use.Fluoroquinolone resistance is an increasing problem not only
in the U.S. but also worldwide, potentially due to the widespread misuse of
this class of antimicrobials. The Interagency Task Force on Antimicrobial
Resistance suggests that enhanced surveillance of antimicrobial resistance is
critical to extend the therapeutic use of antimicrobials, including
fluoroquinolones. In addition, the production of new agents is vital to combat
this mounting rate of resistance. However, until new agents can be produced,
stricter guidelines should be initiated to preserve this broad class of
antibiotics and others as well. Furthermore, the public and health care
providers should be educated on the impact of inappropriate antimicrobial use.
References
1. Ball P. Quinolone generations:
natural history or natural selection? J Antimicrob Chemother.
2000;46:17-24.
2. Bakken JS. The fluoroquinolones:
how long will their utility last? Scand J Infect Dis. 2004;36:85-92.
3. Ciprofloxacin hydrochloride
package insert. West Haven, CT: Bayer Pharmaceuticals Corporation, 2005.
4. Ofloxacin package insert. Raritan,
NJ: Ortho-McNeil Pharmaceutical, Inc., January 2006.
5. Norfloxacin package insert.
Whitehouse Station, NJ: Merck & Co. Inc.
6. Lomefloxacin package insert. New
York, NY: Pfizer, April 2005.
7. Gemifloxacin package insert.
Waltham, MA: Oscient Pharmaceuticals, August 2004.
8. Levofloxacin package insert.
Raritan, NJ: Ortho-McNeil Pharmaceutical, Inc., August 2005.
9. Gatifloxacin package insert.
Princeton, NJ: Bristol-Myers Squibb Co., 2006.
10. Moxifloxacin package insert.
Kenilworth, NJ: Bayer Pharmaceuticals Corporation, 2005.
11. McEvoy GK. AHFS Drug
Information 2005. Bethesda, MD; 2005.
12. Linder JA, Huang ES, et al.
Fluoroquinolone prescribing in the United States: 1995-2002. Am J Med.
2005;118:259-268.
13. Simoens S, Verhaegen J, et al.
Treating respiratory tract infections in ambulatory care in Belgium:
fluoroquinolone consumption and resistance development. Int J Antimicrob
Agents. 2005;26(1):62-68.
14. Trends in antimicrobial drug
development: implications for the future. Clin Infect Dis.
2004;38:1279-1286.
15. Lautenbach E, Strom B, et al.
Longitudinal trends in fluoroquinolone resistance among Enterobacteriaceae
isolates from inpatients and outpatients, 1989-2000: Differences in the
emergence and epidemiology of resistance across organisms. Clin Infect Dis
. 2004;38:655-662.
16. Karaca Y, Coplu N, et al.
Co-trimoxazole and quinolone resistance in Escherichia coli isolated
from urinary tract infections over the last 10 years. Int J of Antimicrob
Agents. 2005;26:75-77.
17. Hooper, DC. Emerging mechanisms
of fluoroquinolone resistance. Emerg Infect Dis. 2001;7:337-341.
18. Ferra AM. New fluoroquinolones in
lower respiratory tract infections and emerging patterns of pneumococcal
resistance. Infection. 2005; 33:106-114.
19. Wang M, Tran H, et al.
Plasmid-mediated quinolone resistance in clinical isolated of Escherichia
coli from Shanghai, China. Antimicrob Agents Chemother.
2003;47:2242-2248.
20. Interagency Task Force on
Antimicrobial Resistance. A Public Health Action Plan to Combat Antimicrobial
Resistance Part 1: Domestic Issues; 2002.
21. Quale J, Landman D, et al.
Streptococcus pneumoniae, Brooklyn, New York: Fluoroquinolone resistance
at our doorstep. Emerg Infect Dis. 2002; 8:594-597.
22. Ho P, Que T, et al.
Fluoroquinolone and other antimicrobial resistance in invasive pneumococci,
Hong Kong, 1995-2001. Emerg Infect Dis. 2004;10:1250-1257.
23. Yamaguchi K, Ohno A, Levofloxacin
Surveillance group. Investigation of the susceptibility trends in Japan to
fluoroquinolones and other antimicrobial agents in a nationwide collection of
clinical isolates: a longitudinal analysis from 1994 to 2002. Diagn
Microbiol Infect Dis. 2005;52:135-143.
24. Bastida T, Perez-Vazquez M, et
al. Levofloxacin treatment failure in Haemophilus influenzae pneumonia.
Emerg Infect Dis. 2003;9:1475-1478.
25. Chomarat M. Resistance of
bacteria in urinary tract infections. Int J Antimicrob Agents.
2000;16:483-487.
To comment on this article, contact editor@uspharmacist.com.






