US Pharm. 2006;HS42-HS50.
By 2010, the cost of diabetes care--including screening, prevention, and treatment programs--is expected to reach $156 billion. Pharmacotherapy--including insulin, its delivery systems and supplies, and oral antidiabetic agents--represents 13% of diabetes-related expenditures.1 Economic studies have firmly established the value of intensive glycemic control, reporting reduced morbidity and mortality and improved quality of life.2-6 One study estimated that over 10 years, cost savings of between $50 billion and $72 billion--or 4% to 6% of health care expenditures per year--would accrue if hemoglobin A1c (HbA1c) is maintained at a level of at least 7% or 6.5%, respectively.7 Other studies have shown that with proper education and management addressing hypoglycemia and weight gain, adding insulin to type 2 diabetes regimens may decrease health care costs significantly in as little as two months.2,8-10 Since increased body weight also affects cardiovascular disease, hyperlipidemia, and hypertension--each with its own economic implications--prevention of weight gain should exert long-term cost savings.
The pharmacokinetic and pharmacodynamic properties of insulin analogs, such as aspart, lispro, glulisine, glargine, and detemir, as well as premixed analogs, such as biphasic insulin aspart [BIAsp] 70/30 (containing a mixture of 70% protaminated and 30% soluble insulin aspart) and lispro 75/25 (containing 75% insulin lispro protamine suspension and 25% insulin lispro) provide more physiological and reliable time-action profiles than regular human insulin (RHI) or neutral protamine Hagedorn (NPH) insulin and represent a significant advancement in diabetes management.11,12 Compared with older insulin formulations, insulin analogs provide dosing flexibility, which may improve patient satisfaction and adherence to therapy, and are associated with a lower risk of hypoglycemia and better postprandial and fasting glycemic control.11-20 In addition, long-acting insulin analogs, particularly insulin detemir, are associated with less weight gain than is NPH insulin.13,20,21 Together, these characteristics may contribute to health care cost benefits.
Intensive Diabetes Management Improves Health
Outcomes
Achieving and maintaining a lower
HbA1c level reduces morbidity and mortality in patients with diabetes.
22,23 Therefore, the American Diabetes Association (ADA) has recommended
a therapeutic HbA1c target of less than 7% for individuals with diabetes,
while the American Association of Clinical Endocrinologists recommends a
target of 6.5% or lower.24,25
Unfortunately, fewer than 25% of patients who have had type 2 diabetes for a nine-year duration are adequately controlled with sulfonylureas or metformin, and 60% to 68% have HbA1c values of 8% or greater. 22,26 As oral medications fail, the patient, physician, and other members of the diabetes care team must work together to overcome fear of hypoglycemia and weight gain so that insulin may be initiated earlier and adherence to therapy improved.27
Pharmacokinetic and Pharmacodynamic Advantages
of Insulin Analogs
The pharmacokinetic and
pharmacodynamic profiles of rapid-acting (i.e., insulins aspart, glulisine,
and lispro) and long-acting insulin analogs (i.e., insulins detemir and
glargine) offer patients more flexibility and predictability than RHI and NPH
insulin (see table 1).11 When used in basal-bolus
combination regimens or in premixed formulations (i.e., BIAsp 70/30 or insulin
lispro 75/25), insulin analogs mimic the physiological insulin response with
minimal variability.19,21,28,29
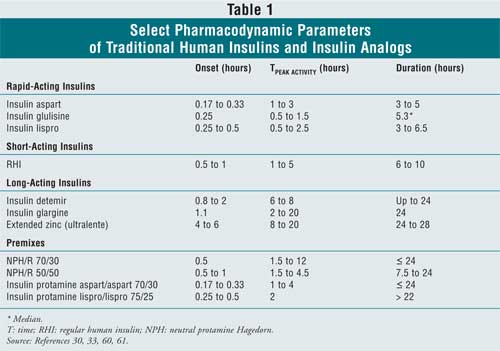
Rapid-Acting Insulin Analogs: Because rapid-acting insulin analogs are quickly absorbed and begin acting within five to 15 minutes, insulins aspart, glulisine, lispro, and the analog premixes may be injected immediately before or soon after a meal.12,19 Peak insulin concentrations are attained within 30 to 90 minutes to coincide with the peak postprandial glucose load. Rapid-acting insulin analogs are also cleared within three to six hours, reducing the likelihood of between-meal or nocturnal hypoglycemia. In contrast, RHI circulates for six to 10 hours, and the time to maximum concentration can be prolonged by 62% when the dose is doubled.28,30 Neither the speed of onset nor the duration of glycemic effects of insulin aspart or lispro is appreciably increased with dose.28,31
Rapid-acting insulin analogs are at least as effective as RHI in maintaining overall glycemic control, with similar or lower risk of hypoglycemia.31-34 They offer a key advantage by controlling postprandial glucose, a well-recognized risk factor for cardiac disease and death in patients with diabetes.35 Several comparative randomized clinical trials in patients with both type 1 and type 2 diabetes have shown that compared to patients treated with RHI, lispro, aspart, and glulisine all demonstrated better postprandial glucose control without increasing the risk of hypoglycemia.14,17,18,33,34
One large crossover study demonstrated 11% fewer hypoglycemic episodes with insulin lispro compared with RHI (P < .001), with the largest relative between-treatment difference occurring at night. 36 In a review using pooled data from patients with type 1 diabetes, nocturnal hypoglycemia occurred 38% less frequently in patients receiving insulin aspart than in patients receiving RHI.32 Severe hypoglycemia occurred much less frequently in patients with type 2 diabetes and was even less likely to occur with insulin analogs than with RHI (median 0.6 vs. 2.8 episodes per 100 patient-years, respectively).34
Long-Acting Insulin Analogs: Long-acting insulin analogs were designed to better mimic the constant basal insulin component of physiological insulin that moderates fasting plasma glucose. Exhibiting relatively flat and prolonged pharmacokinetic profiles after subcutaneous injection, these analogs improve upon the unpredictable peaks and within-patient variability observed with NPH or ultralente insulin, enabling up to 24-hour glycemic control while reducing hypoglycemia risk, possibly allowing for more aggressive titration of insulin doses. 13,15,20,21,37
Insulin glargine's effects are due to delayed release from amorphous microprecipitates that form in subcutaneous tissue upon injection.20 Healthy individuals treated with insulin glargine exhibited lower 24-hour within-patient variability than did those treated with ultralente and NPH insulin.38 Insulin glargine is usually administered once daily and produces greater reductions in fasting plasma glucose and similar or better HbA1c reductions than twice-daily NPH insulin. 20 Its ability to maintain equivalent or improved glycemic control in comparison with NPH insulin has been demonstrated in short- and long-term randomized studies of patients with type 1 diabetes who continue their usual mealtime insulin regimens and patients with type 2 diabetes who remain on concurrent oral antidiabetic therapy.20
Insulin detemir, which remains soluble after subcutaneous injection, owes its slow absorption properties to a combination of self-association of hexamers at the injection site and reversible binding to albumin (98% to 99% bound) in subcutaneous tissue and plasma.13 This albumin binding gives rise to an important buffering effect that limits pharmacodynamic variability, but with no clinically relevant interactions between detemir and free fatty acids or protein-bound drugs.13 In several randomized trials, insulin detemir provided more predictable glucose-lowering effects and less day-to-day variation than NPH insulin, and in one randomized comparison with insulin glargine and NPH, insulin detemir demonstrated the lowest within-subject pharmacodynamic variability.21,39 When given to patients with type 1 diabetes as part of a basal-bolus regimen, once- or twice-daily insulin detemir resulted in lower fasting plasma glucose levels after six months, lower prebreakfast glucose levels, and lower day-to-day variability in self-monitored blood glucose levels with HbA1c reductions comparable to those with NPH insulin.21 In patients with type 2 diabetes, once- or twice-daily insulin detemir combined with insulin aspart in basal-bolus therapy or oral antidiabetic agents provided comparable glycemic control to basal-bolus NPH insulin plus RHI or NPH insulin plus oral antidiabetic agents.40,41
Reduced variability of insulin analogs compared with NPH insulin should result in fewer episodes of hypoglycemia, and clinical trials with insulins glargine and detemir confirm this prediction.39 A meta-analysis of four open-label studies comparing insulin glargine to NPH insulin in 2,300 patients with type 2 diabetes estimated that the risk of overall and nocturnal hypoglycemia was reduced by 11% (P = .0006) and 26% (P < .0001), respectively, in patients taking insulin glargine. 15 Once-daily insulin detemir reduced the risk of nocturnal hypoglycemia by 26% compared to once-daily NPH insulin (P = .003) HbA1c reductions were similar with both agents.21 Patients with type 2 diabetes who were treated with both insulin detemir and oral antidiabetic agents had 47% and 55% fewer overall and nocturnal hypoglycemic events, respectively, than did patients treated with NPH insulin and oral antidiabetic agents (P < .001).41
Analog Premixes: Premixed biphasic insulin analogs offer similar pharmacokinetic and pharmacodynamic advantages to those of long-acting and rapid-acting insulin analogs. Premixed analogs are an excellent option for patients with type 2 diabetes who seek simplicity, minimal injections, and convenience, as well as fasting plasma glucose and postprandial glucose control without additional hypoglycemia risk.19 The faster absorption rate of biphasic insulin lispro 75/25 contributed to better postprandial glucose control relative to human insulin 70/30 (a mixture of 30% RHI and 70% NPH insulin) in clinical trials involving patients with type 1 and type 2 diabetes.19 In long-term studies evaluating overall glycemic control, insulin lispro 75/25 resulted in similar HbA1c reductions and better fasting plasma glucose control compared with human 70/30 premix, with no significant differences in hypoglycemia.19
Similarly, BIAsp 70/30 has a more rapid onset, providing better postprandial glucose control, comparable reductions in HbA1c, and similar hypoglycemia incidence, compared with human 70/30 premix.19 The 1-2-3 Study employed a treat-to-target approach in patients with type 2 diabetes poorly controlled on oral antidiabetic agents. This study reported that once-, twice-, or three-times-daily BIAsp 70/30 resulted in 41%, 70%, and 77% of patients, respectively, achieving ADA glycemic targets (< 7%), respectively.42†
Insulin Analogs and Weight Gain: Obesity is an important risk factor for cardiovascular disease; therefore, weight gain is a genuine concern for patients who are using (or considering) insulin to achieve intensive glycemic control. Physical activity and caloric intake are key modifiable factors to address when any insulin therapy is initiated to compensate for glucose retention and more efficient metabolism resulting from better glycemic control.27 Insulin analogs offer some benefit to patients who gain weight due to fear of hypoglycemia, possibly because the perceived risk of hypoglycemia with these treatments is reduced. 43
Weight gain is less problematic with long-acting insulin analogs than with NPH insulin. In particular, patients treated with insulin detemir have consistently demonstrated significantly less weight gain than those treated with NPH insulin.21 In type 2 populations (in whom weight gain is most problematic),† more favorable effects on weight were observed when basal insulin detemir was combined with mealtime insulin aspart compared to NPH/RHI (0.5 vs. 1.1 kg; P = .038) and also when combined with oral antidiabetic agents compared to NPH/oral antidiabetic agents (1.2 vs. 2.8 kg; P < .001).40,41 One six-month comparative study reported that patients with type 2 diabetes demonstrated less weight gain with insulin glargine (0.4 kg vs. 1.4 kg; P = .0007) than with NPH insulin; however, the difference was not sustained after 12 months.20,44
Pharmacoeconomic Impact of Insulin Analogs
Containing costs for diabetes
involves more than just minimizing up-front spending. In 2002, insulin
(including delivery systems and supplies) and oral antidiabetic agents
accounted for just 7.6% and 5.4% of the total expenditures on diabetes care,
respectively.1 If insulin analogs can improve outcomes, an enormous
opportunity awaits to curtail the largest portion of diabetes-related spending
(87%, or ~$80 billion) that is attributed to hospital stays, physician visits,
hospice care, home health, management of comorbidities, and lost productivity.
Additionally, better glycemic control may translate into improved quality of
life for patients and their families.
What pharmacoeconomic advantages of insulin analogs can be expected? Three separate but highly interdependent pathways to health care cost savings may be envisioned. First, savings will be realized through prevention of diabetes-related complications, due to better glycemic control; second, savings will accrue through better therapy adherence; and third, a reduced need to treat therapy-related complications will result in fewer emergency department visits and hospitalizations and lower costs.
Improved Glycemic Control: Intensive insulin therapy, as practiced in the Diabetes Control and Complications Trial, was more costly by $28,661 per year of life saved than was conventional management, but it represents a good value from a health care system perspective.23,45 In a managed care setting, with higher charges associated with increasing HbA1c and cardiac morbidities, health care costs for diabetes could be trimmed by $400 to $4,000 per person over a three-year period for each 1% reduction in HbA1c.6 Shetty et al. reported that patients achieving HbA1c 7% or lower incurred 32% lower health care costs in the follow-up year than when this target was not met.3 In another example, achieving glycemic control improved symptoms, general perceived health, and cognitive functioning, translating to average savings of $91 per worker per month attributable to less absenteeism, $304 per 1,000 person-days attributable to fewer hospital-bed-days, and $1,615 per 1,000 person-days attributable to reduced restricted-activity-days.4 Improving HbA1c by 1% or more for sustained periods eventually saved one health maintenance organization between $685 and $950 per patient per year, despite higher costs for primary care visits in the first year.5
A meta-analysis of four trials that used insulin detemirñbased basal/bolus regimens yielded an incremental cost-effectiveness ratio of $36,284 (converted from £; September 2006) per quality-adjusted life-years (QALY) gained, a good value by international health care standards. 46 In another health economic evaluation of insulin glargine and NPH insulin that projected lifetime direct drug and complications costs, insulin glargine was found to be clinically beneficial and more cost-effective than NPH insulin. Although NPH insulin was less expensive, insulin glargineñtreated patients experienced less hypoglycemia at lower HbA1c values, gaining 0.07 QALY at $23,717 per QALY and 0.23 QALY at $9,804 per QALY in patients with type 1 and type 2 diabetes, respectively.47
Complications from cardiovascular disease account for a large portion of health care costs in patients with diabetes. With evidence mounting that postprandial glucose is an important heart disease risk factor, postprandial glucose control is a major opportunity for rapid-acting insulin analogs to lower health care costs relative to RHI; however, these cost savings have yet to be documented in well-controlled studies.35,48
Improved Adherence to Therapy: Adherence to pharmacotherapy is crucial to controlling disease and its sequelae. Estimated percentages of patients with poor adherence to diabetes therapy (insulin, oral antidiabetic agents, or both) range from 36% to 93%. 49 Fear of hypoglycemia and weight gain, depression, needle aversion (in the case of insulin therapy), or insufficient motivation arising from a lack of education about the benefits and options for self-management can result in poor adherence and can negatively affect glycemic control, long-term outcomes, and mortality.27,50,51
Observational studies have confirmed that adherence to pharmacotherapy lowers disease-related medical costs. Analyzing diabetes patients by five levels of adherence showed that the lowest diabetes-related hospitalization risk (13%) was observed in the most adherent patients, whereas in less adherent patients, hospitalization rates were 20% to 30% (P < .001; chi-square).52 Although drug costs were higher at each adherence level, medical costs decreased from $15,186 (least) to $6,377 (most) during the 12-month observation period. A five-year study of patients with type 2 diabetes found that for each 10% increase in oral antidiabetic medication adherence, an 8.6% reduction in annual health care costs (P< .001) was attained.53 In the corporate environment, high-cost medical claims were frequently filed by less compliant patients, i.e., those who refilled their insulin or oral antidiabetic prescriptions less than two thirds of the time. Changing the prescription drug benefit to include insulin analogs among drug choices resulted in better adherence to therapy, 26% lower emergency service utilization, and 7% lower pharmacy costs for diabetes patients.54
No studies have yet directly addressed whether insulin analogs are associated with better adherence and lower costs than human insulins. However, reducing fear actually drives better fasting plasma glucose control, as recently demonstrated in patients with type 2 diabetes who self-titrated insulin doses to achieve fasting plasma glucose less than 100 mg/dL (5.5 mmol/L). More patients who self-treated with insulin glargine than those treated with NPH insulinwere inclined to adjust their doses to achieve fasting plasma glucose targets.37 These findings indicate† that the reduced risk of hypoglycemia and weight gain, as well as the flexibility and predictability associated with insulin analogs, may minimize patients' aversion to insulin therapy, improve acceptance and adherence, and ultimately contribute to lower medical costs.
Lower Rates of Adverse Events: Increased utilization of health care resources and lower productivity are associated with adverse consequences of intense glycemic control.2,8,10,55 One such complication is moderate/severe hypoglycemia which is approximately tenfold more common in patients with type 1 and type 2 diabetes.56 Heaton et al. reported that the overall mean cost per moderate/severe hypoglycemic episode ranged from $181 to $4,924 (average, $1,186), depending on whether treatment was provided at a doctor's office or hospital.8 In a study where the annualized cost of hypoglycemia was $3,241 per patient, twice as many emergency department visits and hospitalizations and 77% more short-term disability days were noted in patients treated for hypoglycemia. 10 In one population-based study of 115 patients with type 2 diabetes in which insulin was used by 43% of patients and hypoglycemia incidence was 37%, direct health care costs were $82.90 per severe hypoglycemic episode and $12.90 per patient with hypoglycemic symptoms per month. Lost work time added $14.10 per symptomatic patient per month.55 Although no hospitalizations were recorded, a single hospitalization for hypoglycemia typically costs nearly $5,000, and a day of sick leave costs approximately $200; even one hospital admission (assuming several lost workdays) would have raised these cost estimates considerably.8,55 Using data from both Sieberhofer et al.'s meta-analysis (which estimated a median of 17 fewer severe hypoglycemic episodes per 100 person-years with insulin analogs versus RHI) and Heaton et al.'s average cost per episode of hypoglycemia of $1,186, savings of $20,162 per 100 person-years can be expected.8,34 Patients with type 2 diabetes treated with insulin analogs had 2.2 fewer hypoglycemic episodes per 100 person-years than did those treated with RHI, translating to a potential cost savings of $2,609.34 Since type 2 diabetes represents 90% to 95% of all diabetes cases,57 the cost-saving potential with reduced hypoglycemia is enormous.
One pharmacoeconomic study documented that for every nine patients treated with insulin glargine versus NPH insulin, one hypoglycemic event would be avoided; the extra cost of glargine was more than offset by costs to manage the event.16 A pooled analysis of patients with type 1 diabetes revealed that those who received insulin detemir had 22% fewer hypoglycemic episodes than did those treated with NPH insulin at comparable HbA1c levels (P < .001).58 In patients with type 2 diabetes uncontrolled with oral antidiabetic agents, hypoglycemia occurred 47% less frequently with insulin detemir than with NPH insulin (P < .001).41 Based on a crude first estimate, using insulin detemir for basal replacement could save $4,000 to $8,500 per 100 patients (i.e., 22 to 47 patients avoiding moderate to severe hypoglycemia at $181 to $4,924 per episode), compared with using NPH insulin.
With regard to weight gain, one recent study found that overweight or obese persons older than 70 add, in costs to society, $13,000 to $39,000 more (i.e., Medicare, individuals, private insurers, Medicaid, and other non-Medicare insurers) than do normal-weight persons. 59 In another study, patients who were able to achieve strict glycemic goals (HbA1c < 6.5%) without gaining weight had a longer life expectancy (by 0.57 year), higher QALY (by 0.28 year), and lower costs due to complications (by $523 per patient) than patients who gained weight on intensive therapy. 9 The weight-neutral effects of insulin detemir, in comparison with the weight gain observed with NPH insulin, can help diminish concerns about weight gain and its related costs in patients with diabetes.
Summary and Conclusions
Tight glycemic control in
patients with diabetes results in health and economic benefits. However, tight
control may be difficult to achieve and maintain with human insulins. Fear of
hypoglycemia and weight gain may contribute to poor adherence, leading to a
cycle of suboptimal control and escalating health care costs. In today's
managed care environment, pharmacists are challenged to select therapies that
minimize costs and therapy-related morbidities. Insulin analogs more closely
replicate normal physiological patterns of insulin secretion, exhibit less
within-patient variability, and offer added flexibility and safety compared to
human insulin formulations. Studies quantifying the pharmacoeconomic benefits
of insulin analogs are necessary, but given these improvements, better therapy
adherence and cost benefits should begin to emerge.
REFERENCES
1. American Diabetes Association.
Economic costs of diabetes in the U.S. in 2002. Diabetes Care.
2003;26:917-932.
2. Rosenblum MS, Kane MP. Analysis of
cost and utilization of health care services before and after initiation of
insulin therapy in patients with type 2 diabetes mellitus. J Manag Care
Pharm. 2003;9:309-316.
3. Shetty S, Secnik K, Oglesby AK.
Relationship of glycemic control to total diabetes-related costs for managed
care health plan members with type 2 diabetes. J Manag Care Pharm.
2005;11:559-564.
4. Testa MA, Simonson DC. Health
economic benefits and quality of life during improved glycemic control in
patients with type 2 diabetes mellitus: a randomized, controlled, double-blind
trial. JAMA. 1998;280:1490-1496.
5. Wagner EH, Sandhu N, Newton KM, et
al. Effect of improved glycemic control on health care costs and utilization.
JAMA. 2001;285:182-189.
6. Gilmer TP, O'Connor PJ, Manning
WG, Rush WA. The cost to health plans of poor glycemic control. Diabetes
Care. 1997;20:1847-1853.
7. Minshall ME, Roze S, Palmer AJ, et
al. Treating diabetes to accepted standards of care: a 10-year projection of
the estimated economic and health impact in patients with type 1 and type 2
diabetes mellitus in the United States. Clin Ther. 2005;27:940-950.
8. Heaton A, Martin S, Brelje T. The
economic effect of hypoglycemia in a health plan. Manag Care Interface.
2003;16:23-27.
9. Palmer AJ, Roze S, Valentine WJ,
et al. Deleterious effects of increased body weight associated with intensive
insulin therapy for type 1 diabetes: increased blood pressure and worsened
lipid profile partially negate improvements in life expectancy. Curr Med
Res Opin. 2004;20 Suppl 1:S67-S73.
10. Rhoads GG, Orsini LS, Crown W, et
al. Contribution of hypoglycemia to medical care expenditures and short-term
disability in employees with diabetes. J Occup Environ Med.
2005;47:447-452.
11. Hirsch IB. Insulin analogues.
N Engl J Med. 2005;352:174-183.
12. Oiknine R, Bernbaum M, Mooradian
AD. A critical appraisal of the role of insulin analogues in the management of
diabetes mellitus. Drugs. 2005;65:325-340.
13. Chapman TM, Perry CM. Insulin
detemir: a review of its use in the management of type 1 and 2 diabetes
mellitus. Drugs. 2004;64:2577-2595.
14. Vazquez-Carrera M, Silvestre JS.
Insulin analogues in the management of diabetes. Methods Find Exp Clin
Pharmacol. 2004;26:445-461.
15. Rosenstock J, Dailey G,
Massi-Benedetti M, et al. Reduced hypoglycemia risk with insulin glargine: a
meta-analysis comparing insulin glargine with human NPH insulin in type 2
diabetes. Diabetes Care. 2005;28:950-955.
16. Bullano MF, Al-Zakwani IS, Fisher
MD, et al. Differences in hypoglycemia event rates and associated
cost-consequence in patients initiated on long-acting and intermediate-acting
insulin products. Curr Med Res Opin. 2005;21:291-298.
17. Bretzel RG, Arnolds S, Medding J,
Linn T. A direct efficacy and safety comparison of insulin aspart, human
soluble insulin, and human premix insulin (70/30) in patients with type 2
diabetes. Diabetes Care. 2004;27:1023-1027.
18. Chapman TM, Noble S, Goa KL.
Spotlight on insulin aspart in type 1 and 2 diabetes mellitus. Treat
Endocrinol. 2003;2:71-76.
19. Garber AJ. Premixed insulin
analogues for the treatment of diabetes mellitus. Drugs. 2006;66:31-49.
20. Dunn CJ, Plosker GL, Keating GM,
et al. Insulin glargine: an updated review of its use in the management of
diabetes mellitus. Drugs. 2003;63:1743-1778.
21. Home P, Kurtzhals P. Insulin
detemir: from concept to clinical experience. Expert Opin Pharmacother.
2006;7:325-343.
22. Intensive blood-glucose control
with sulphonylureas or insulin compared with conventional treatment and risk
of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective
Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853.
23. The effect of intensive treatment
of diabetes on the development and progression of long-term complications in
insulin-dependent diabetes mellitus. The Diabetes Control and Complications
Trial Research Group. N Engl J Med. 1993;329:977-986.
24. American Association of Clinical
Endocrinologists. The American Association of Clinical Endocrinologists
medical guidelines for the management of diabetes mellitus: the AACE system of
intensive diabetes self-management--2002 update. Endocr Pract.
2002;8(suppl 1):40-82.
25. American Diabetes Association.
Standards of medical care in diabetes. Diabetes Care. 2005;28(suppl
1):S4-S36.
26. Palumbo PJ. The case for insulin
treatment early in type 2 diabetes. Cleve Clin J Med. 2004;71:385-386,
391-392, 394 passim.
27. Korytkowski M. When oral agents
fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord
. 2002;26 Suppl 3:S18-S24.
28. Gin H, Hanaire-Broutin H.
Reproducibility and variability in the action of injected insulin. Diabetes
Metab. 2005;31:7-13.
29. Gerich JE. Insulin glargine:
long-acting basal insulin analog for improved metabolic control. Curr Med
Res Opin. 2004;20:31-37.
30. American Society of Health-System
Pharmacists. Antidiabetic agents. In: American Hospital Formulary Service
Drug Information. Bethesda, MD: American Society of Health-System
Pharmacists; 2006:3072-3149.
31. Heller S. Insulin lispro: a
useful advance in insulin therapy. Expert Opin Pharmacother.
2003;4:1407-1416.
32. Chapman TM, Perry CM. Spotlight
on insulin detemir in type 1 and 2 diabetes mellitus. BioDrugs.
2005;19:67-69.
33. Cox SL. Insulin glulisine.
Drugs Today (Barc). 2005;41:433-440.
34. Siebenhofer A, Plank J, Berghold
A, et al. Short acting insulin analogues versus regular human insulin in
patients with diabetes mellitus. Cochrane Database Syst Rev.
2006;CD003287.
35. Gerich JE. Clinical significance,
pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med
. 2003;163:1306-1316.
36. Anderson JH Jr, Brunelle RL,
Koivisto VA, et al. Reduction of postprandial hyperglycemia and frequency of
hypoglycemia in IDDM patients on insulin-analog treatment. Multicenter Insulin
Lispro Study Group. Diabetes. 1997;46:265-270.
37. Fritsche A, Haring HU, Togel E,
Schweitzer M-A. Treat-to-target with add-on basal insulin: can insulin
glargine reduce the barrier to target attainment? [abstract]. Diabetes.
2003;52(suppl 1):A119.
38. Scholtz HE, Pretorius SG, Wessels
DH, Becker RH. Pharmacokinetic and glucodynamic variability: assessment of
insulin glargine, NPH insulin and insulin ultralente in healthy volunteers
using a euglycaemic clamp technique. Diabetologia. 2005;48:1988-1995.
39. Heise T, Nosek L, Ronn BB, et al.
Lower within-subject variability of insulin detemir in comparison to NPH
insulin and insulin glargine in people with type 1 diabetes. Diabetes.
2004;53:1614-1620.
40. Raslova K, Bogoev M, Raz I, et
al. Insulin detemir and insulin aspart: a promising basal-bolus regimen for
type 2 diabetes. Diabetes Res Clin Pract. 2004;66:193-201.
41. Hermansen K, Davies M, Derezinski
T, et al. A 26-week, randomized, parallel, treat-to-target trial comparing
insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering
drugs in insulin-naive people with type 2 diabetes. Diabetes Care.
2006;29:1269-1274.
42. Garber AJ, Wahlen J, Wahl T, et
al. Attainment of glycaemic goals in type 2 diabetes with once-, twice-, or
thrice-daily dosing with biphasic insulin aspart 70/30 (The 1-2-3 study).
Diabetes Obes Metab. 2006;8:58-66.
43. Thompson CJ, Cummings JF,
Chalmers J, et al. How have patients reacted to the implications of the DCCT?
Diabetes Care. 1996;19:876-879.
44. Rosenstock J, Schwartz SL, Clark
CM Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of
insulin glargine (HOE 901) and NPH insulin. Diabetes Care.
2001;24:631-636.
45. Lifetime benefits and costs of
intensive therapy as practiced in the diabetes control and complications
trial. The Diabetes Control and Complications Trial Research Group. JAMA
. 1996;276:1409-1415.
46. Palmer AJ, Roze S, Valentine WJ,
et al. Cost-effectiveness of detemir-based basal/bolus therapy versus
NPH-based basal/bolus therapy for type 1 diabetes in a UK setting: an economic
analysis based on meta-analysis results of four clinical trials. Curr Med
Res Opin. 2004;20:1729-1746.
47. Thompson M, Sauriol L, Grima D.
Health economic evaluation of insulin glargine for the treatment of type-1 and
type-2 diabetes [abstract]. Value Health. 2005;8:A163.
48. Nathan DM, Cleary PA, Backlund
JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes
Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive
diabetes treatment and cardiovascular disease in patients with type 1
diabetes. N Engl J Med. 2005;353:2643-2653.
49. Cramer JA. A systematic review of
adherence with medications for diabetes. Diabetes Care.
2004;27:1218-1224.
50. Krapek K, King K, Warren SS, et
al. Medication adherence and associated hemoglobin A1c in type 2 diabetes.
Ann Pharmacother. 2004;38:1357-1362.
51. Kuo YF, Raji MA, Markides KS, et
al. Inconsistent use of diabetes medications, diabetes complications, and
mortality in older Mexican Americans over a 7-year period: data from the
Hispanic established population for the epidemiologic study of the elderly.
Diabetes Care. 2003;26:3054-3060.
52. Sokol MC, McGuigan KA, Verbrugge
RR, Epstein RS. Impact of medication adherence on hospitalization risk and
healthcare cost. Med Care.2005;43:521-530.
53. Balkrishnan R, Rajagopalan R,
Camacho FT, et al. Predictors of medication adherence and associated health
care costs in an older population with type 2 diabetes mellitus: a
longitudinal cohort study. Clin Ther. 2003;25:2958-2971.
54. Mahoney JJ. Reducing patient drug
acquisition costs can lower diabetes health claims. Am J Manag Care.
2005;11:S170-S176.
55. Lundkvist J, Berne C, Bolinder B,
Jonsson L. The economic and quality of life impact of hypoglycemia. Eur J
Health Econ. 2005;6:197-202.
56. Cryer PE, Davis SN, Shamoon H.
Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902-1912.
57. Centers for Disease Control and
Prevention. National diabetes fact sheet. United States, 2005: general
information. Available at: www.cdc.gov/diabetes/pubs/pdf/ndfs_2005.pdf.
Accessed August 30, 2006.
58. Heller S, Kim H. Insulin detemir
reduces hypoglycemic risk at comparable HbA1c values compared to NPH insulin
in patients with type 1 diabetes [abstract]. Diabetes. 2005;54.
59. Lakdawalla DN, Goldman DP, Shang
B. The health and cost consequences of obesity among the future elderly.
Health Aff (Millwood). 2005;24 Suppl 2:W5R30-W5R41.
60. Novo Nordisk. Levemir(r) (insulin
detemir [rDNA origin] injection) [product information]. Princeton, NJ: Novo
Nordisk A/S. June 16, 2005.
61. Apidra(r) (Insulin Glulisine
[rDNA origin] injection [Product Information]. Kansas City, MO: Aventis
Pharmaceuticals, Inc. November 2005.
To comment on this article, contact editor@uspharmacist.com.






