US Pharm . 2006;8:66-76.
Many pharmacists are aware of the growing popularity and use of herbal medications among patients in the United States. Not only do pharmacists witness sales of these products; they are charged with navigating product selection for self-medicating patients, assessing for safety and drug interactions in patients who are also taking allopathic medications, and encouraging patient communication of such use to all prescribers and health care practitioners involved with their care. Of particular concern are cardiovascular patients, who inevitably take a number of allopathic medications that have demonstrated such outcomes as extending life and minimizing morbidity associated with cardiac disease. When these patients seek complementary and alternative medicine (CAM), either as augmentation or alternatives to their prescription heart medications, pharmacists must be armed with appropriate information about the drug-herb interactions and other safety concerns that may result.
USE OF CAM IN THE U.S.
A 1997 survey estimated that 15
million Americans are at risk for drug-herb interactions due to their
concomitant use of prescription medications and CAM. Three million of those
individuals are 65 and older, making them more likely to have comorbidities
and to partake in polypharmacy. As a result, this population is especially
vulnerable to drug-herb interactions.1 Another study of patients
with acute coronary syndromes reported that nearly 20% of those surveyed took
herbal medications for cardiac-specific purposes.2
Approximately 70% of individuals who use herbal medications do not report such use to their prescribers and pharmacists.3 Unless practitioners learn techniques to properly obtain a complete medication history, including use of CAM therapies, monitoring and recognizing drug-herb interactions will remain an insurmountable barrier in patient care. When asked to disclose all medications taken, patients often consider only prescription drugs obtained in the U.S. Important information may be brought out from asking about not only OTC drugs and CAM therapies but also products purchased from other countries, over the Internet, or via mail order. The patient interview must take place in a nonjudgmental manner that secures trust from the patient, is congruent with or empathetic to his or her cultural beliefs about CAM, and documents such use in the patient's medication databases, so that a vigilant drug interaction screen can be performed.
REGULATORY AND SAFETY ISSUES WITH HERBAL CAM
THERAPY
Most pharmacists are familiar
with some of the challenges and controversies surrounding CAM, particularly
herbal use. Of note are reported adulterants (e.g., heavy metals and legend
drugs),4,5 potential for misbranding (several reports have noted
significant differences between actual ingredients and listed ingredients in
marketed herbal products),6,7 and the limited regulation of these
products by the Dietary Supplement Health and Education Act of 1994. This
legislation places on the government the burden of proving marketed dietary
supplements to be unsafe or ineffective. Dietary supplements may not include
health claims (e.g., diagnosing, treating, curing, or preventing a disease)
and are regulated as food products rather than pharmaceuticals.8
This places tremendous responsibility on individual patients and health care
practitioners to report adverse effects and suspected interactions to the FDA
MedWatch program to ensure that patient safety is accurately assessed.
MedWatch reports may be completed online at
www.accessdata.fda.gov/scripts/medwatch. Additionally, a paper copy of the
MedWatch form may be found in the Physician's Desk Reference, which is
commonly available in medical practices and pharmacy libraries.9
Adverse events may also be voluntarily reported via phone at (800) 332-1088.
Table 1 describes steps a pharmacist should take to manage and report
drug-herb interactions.

Although there has been no fail-safe solution to the issues surrounding product labeling and adulteration, strides have been made toward improving labeling so that consumers and pharmacists have more information and standardized content on OTC dietary supplement packages. The Supplement Facts label resembles the Drug Facts label that most pharmacists and consumers are familiar with. This uniform format requires that dietary supplement ingredients be listed as "amount per serving," with the manufacturers determining the specific serving sizes. Each ingredient in proprietary blends must be listed in descending order by weight. Botanicals must be listed using the common or usual name, and the specific part of the plant used must be stated as well.10 Although these regulations still provide no guarantee of batch-to-batch quality and consistency, they are a step in the right direction of giving consumers and health care practitioners some guidance when selecting dietary supplement products. Likewise, there is no requirement for testing the quality or purity of a product's ingredients (or even whether contents match the label), but several entities have attempted to test the quality and consistency of products' contents. The U.S. Pharmacopeia Dietary Supplement Verification Program (USP-DSVP),11 the National Sanitation Foundation (NSF) International Good Manufacturing Practices Program, and the ConsumerLab (CL) Voluntary Certification Program12 test dietary supplements to determine if the label accurately indicates contents, that no common contaminants such as pesticides or heavy metals are included, and that the tablets or capsules undergo proper dissolution. USP-DSVP additionally ensures that the products are made in facilities that meet standards for good manufacturing practices. Each entity offers a seal of approval, indicating that the product "passed" testing. However, CL offers the seal only to companies that are willing to pay for rights to display the seal on their label. Pharmacists and consumers can look for these seals of approval as one means of selecting products that have at least met the quality standards of USP, NSF, or CL.
DATA REGARDING SPECIFIC DRUG-HERB INTERACTIONS
Historically, allopathic
practitioners have determined that studies are often lacking or poorly
conducted in the area of herbal therapy, making it difficult to embrace
alternatives as part of mainstream therapy. Variability in growing conditions,
specific parts of the plants used, and manufacturing procedures create further
difficulty in selecting appropriate products when convincing data are present
for a particular herb.
Despite such barriers, practitioners must educate themselves about herbal use and safety, because patients choose to use CAM therapies regardless of health care professional involvement or recommendation. While there are a great deal of efficacy and safety studies on herbs such as St. John's wort or dietary supplements such as omega-3 fatty acids, there are few well-conducted studies on the efficacy and safety of other, less-known CAMs; therefore, in attempting to interpret drug-herb interactions, the medical community has only case reports and theories based on mechanisms of action or metabolism to refer to. This review details both reported and theoretical drug-herb interactions with prescription cardiac drugs based on available data. These interactions are listed alphabetically by herb, under each respective cardiac drug class.
Anticoagulant and Antiplatelet Drugs
A myriad of herbal therapies have
shown real or potential interactions with anticoagulants, such as warfarin,
and antiplatelet drugs, such as NSAIDs, clopidogrel, and aspirin. Table 2
displays these herbs along with the suspected or reported therapeutic
alteration.
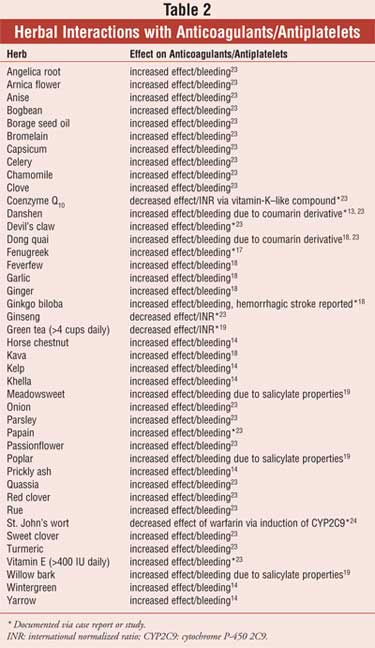
Horse Chestnut: Pharmacists may see horse chestnut used for venous insufficiency and varicose veins. Reductions in lower leg edema following use of horse chestnut were similar to those experienced with use of compression stockings. There is theoretical concern that horse chestnut may increase effects of antiplatelets and anticoagulants due to its coumarin component and antiplatelet properties. 13,14
Antihypertensives
Aconite:
This herb affects the voltage-sensitive sodium channels of excitable
membranes and is toxic to both neurons and cardiac cells. By exerting vagal
action, it can cause hypo tension and bradycardia. Although these
effects can be reversed with atropine, they may confound treatment with
antihypertensives.14
Bitter Orange: This herb is included in many "ephedra-free" weight-loss dietary supplement preparations. Similarly to ephedra, bitter orange was recently found to increase blood pressure and heart rate for up to five hours postadministration. These adverse effects may counteract the blood pressure–lowering effects of antihypertensives.15
Danshen: This herb inhibits platelet aggregation and appears to potentiate the effects of warfarin. Danshen has also been reported to antagonize the effects of propranolol, potentially leading to a lessened antihypertensive effect.14
Ephedra (ma huang): This sympathomimetic herb is associated with increased blood pressure, as well as tachycardia, stroke, myocardial infarction, and sudden death. This herb may also cause coronary spasm, with may negate antianginal therapy. Ephedra should be avoided, particularly in patients with cardiovascular disease or hypertension.16 Ephedra was ordered off the market, but a Utah judge recently ruled that ban to be illegal. As a result, this herb may become available once again.
Ginkgo Biloba: Elevated plasma levels of nifedipine have been experienced in a controlled trial using ginkgo biloba. Patients may experience excessive reductions of blood pressure and, possibly, increased adverse effects from nifedipine therapy.17
Ginseng (Asian, American, and Siberian): This herb may cause elevated blood pressure as a side effect, negating the effects of some antihypertensives.16,18 One case report deemed ginseng responsible for reducing the diuretic effect of furosemide. Additional studies of nifedipine and ginseng found nifedipine plasma concentrations to rise, potentially leading to excessive blood pressure reduction and exaggerating the adverse effects of nifedipine.17
Grapefruit Juice: Although grapefruit juice is technically not considered a CAM, it can significantly interfere with prescription drugs; therefore, it is discussed in this article. The flavonoids found in grapefruit juice are known to inhibit cytochrome P-450 3A4 (CYP3A4), thereby increasing the bioavailability of certain calcium channel blockers. This interaction was first discovered with felodipine. Nitrendipine and nisoldipine are also subject to this interaction. Because they are more bioavailable, nifedipine, nimodipine, and amlodipine do not seem to be affected to a clinically significant degree, although plasma levels may be elevated. Likewise, whether nondihydropyridine calcium channel antagonists (such as diltiazem and verapamil) are affected is a controversial topic.19,20
Guggul (Gugulipid): This herb has been shown to reduce the bioavailability (significant reductions in peak concentrations and area under the curve) of propranolol and diltiazem, potentially leading to a reduced response to these antihypertensive agents.13
Hawthorn: This herb, which has been studied in congestive heart failure, may exhibit additive hypotensive effects when used with antihypertensives.13 Theoretically, several active components of hawthorn extract may also increase the risk of bleeding if taken with antiplatelet agents. These active molecules inhibit the biosynthesis of thromboxane A2, the primary mediator of platelet aggregation.
Hellebore: This herb may exhibit antihypertensive effects, lowering blood pressure and slowing heart rate. This may confound antihypertensive therapy, and patients should be carefully monitored for adverse effects.13,18
Juniper Berry: This herb has diuretic properties that may confound antihypertensive therapy. Nephrotoxicity has also been reported with use of juniper berry.19
Licorice: This herb may elevate blood pressure, negating the effects of antihypertensive therapy. Due to its mineralocorticoid activity, licorice may antagonize effects of spironolactone, minimizing its diuretic effect. Pseudoaldosteronism accompanied by weight gain, hypokalemia, hypertension, and metabolic alkalosis has been reported.20,21
Melatonin: This supplement may interfere with nifedi pine therapy, leading to increased blood pressure.17 Melatonin might also increase the risk of bleeding when used with warfarin.
Parsley Oil: This oil has diuretic properties that may confound antihypertensive therapy. Patients should be monitored for excessive hypotension. Nephrotoxicity has also been reported with this oil. 19
Panax Notoginseng ("pseudoginseng"): This herb has been described as having calcium channel blocker activity that may exert additive antihypertensive properties when administered concomitantly. 13
St. John's Wort: This herb may interfere with calcium channel blockers via modulation of P-glycoprotein.22 Additionally, when studied with nifedipine, St. John's wort was found to reduce nifedipine plasma levels.17
Yohimbine: This agent exhibits presynaptic alpha-adrenergic blocking properties and may also inhibit monoamine oxidase. It may cause high blood pressure even when given in doses as low as 10 mg. It has been implicated in at least one case of hypertensive emergency. Because yohimbine may increase norepinephrine, it is especially contraindicated in patients with coronary artery disease and chronic heart failure.20
Cardiac Glycosides
Adonis:
This herb contains cardiac glycosides that may have additive effects when
given with digoxin therapy.13 The most commonly observed effects
following cardiac glycoside plant intoxication may include bradycardia with
arteriovenous block, hypotension, lethargy, dizziness, and gastrointestinal
upset.
Black Hellebore: This herb is considered a plant source of cardiac glycosides and may have additive effects with digoxin, thereby predisposing patients to toxicity. 13 These plant glycosides may produce cardiac toxicity, including sinus bradycardia and escape beats.
Black Indian Hemp: This herb contains cardiac glycosides that may have additive effects when given with digoxin therapy.13
Hawthorn: This herb may demonstrate additive (positive inotropic and negative chronotropic) properties with digoxin.13,18
Kyushin: This herb may interfere with digoxin drug level assays.23 One of the ingredients of kyushin is toad venom toxin, which is cardioactive with properties similar to digitalis. Ingestion or dermal absorption of toad venom has caused typical digitalis-like poisoning, with dysrhythmias, heart block, hypotension, and vomiting.
Oleander: This plant contains cardiac glycosides that may have additive effects when given with digoxin therapy. Toxicity and death have been reported with ingestion of merely one leaf.13
St. John's Wort: This herb has demonstrated reduced digoxin levels resulting from modulation of P-glycoprotein.24 Hypertension may also occur as part of the serotonin syndrome, which may result from the interaction of St. John's wort with a monoamine oxidase inhibitor, a serotonin reuptake inhibitor, or an indirect-acting sympathomimetic drug.
Siberian Ginseng: This herb can increase digoxin drug levels and lead to digoxin toxicity. 25
Wall Flower: This herb contains cardiac glycosides that may have additive effects when given with digoxin therapy.13
Antihyperlipidemics
Chinese Red Yeast Rice:
This yeast contains a compound similar to lovastatin, which may have additive
properties of lipid lowering as well as adverse effects such as myopathy,
rhabdomyolysis, and liver enzyme elevation.19
Echinacea: One study found that prolonged use of echinacea might be hepatotoxic, although this finding was later negated. Such hepatotoxicity could be additive with antihyperlipidemics that exhibit hepatic side effects (e.g., hydroxymethyl glutaryl coenzyme A–reductase inhibitors, fibrates, and nicotinic acid).3
Garlic: This herb may offer additive lipid-lowering effects when large quantities are consumed (five to 20 cloves). Garlic use had resulted in a maximum reduction in total cholesterol by 6.1% and a maximum reduction in LDL cholesterol by 4.6%.13
Grapefruit Juice: Due to inhibition of CYP3A4, drug concentrations of atorvastatin, simvastatin, and lovastatin may increase threefold to ninefold. Not only would enhanced efficacy be expected, but dose-related adverse effects, such as myopathy, rhabdomyolysis, and liver enzyme elevations could also occur.26
Guggul (Gugulipid): This herb can demonstrate additive lipid-lowering effects when used concomitantly with antihyperlipidemic drugs. Guggul lowered LDL cholesterol by as much as 12.5% in one study.13
Peppermint Oil: One study found that peppermint oil might increase serum levels of simvastatin, resulting in increased therapeutic and adverse effects of the drug.17
St. John's Wort: Due to induction of CYP3A4, St. John's wort may decrease levels of atorvastatin, lovastatin, and simvastatin, thus reducing the pharmacological effects of the antihyperlipidemics. Due to its potential to induce CYP2C9, the herb may theoretically decrease levels of fluvastatin and rosuvastatin.17
Antiarrhythmics
Aconite:
Potentially fatal dysrhythmias, including ventricular fibrillation, have been
reported with use of aconite. This potential adverse effect may negate
antiarrhythmic therapy.14 Atrial fibrillation is uncommon.
Echinacea: This herb may inhibit CYP3A4, resulting in QT-interval prolongation, and potentially, torsades de pointes in patients taking amiodarone, cyclosporine, propafenone, ibutilide, or other drugs that are metabolized via CYP3A4 and have the potential for QT prolongation.16,18
Ephedra (ma huang): This herb may lead to tachycardia and erratic heart rhythm and may interfere with antiarrhythmic therapy. Due to fatalities reported with ephedra, it is not recommended its use, particularly in cardiac patients.18
St. John's Wort: This herb may interfere with lidocaine, quinidine, and amiodarone therapy via modulation of P-glycoprotein. St. John's wort has also been found to induce CYP3A4 and CYP2C9.16,18
Yohimbine: This agent may cause dysrhythmias (specifically tachyarrhythmia), thereby interfering with antiarrhythmic therapy.18
Herbs That May Interfere with Cyclosporine
(Applicable to Heart Transplant Patients)
Alfalfa Sprouts:
The immunostimulant properties of alfalfa sprouts may negate the effects of
cyclosporine.3
Astragalus: This herb has immunostimulant properties that may negate the immunosuppressant effects of cyclosporine.3
Echinacea: Continued use of echinacea may lead to transplant rejection if coadministered with cyclosporine due to echinacea's immunostimulant properties.3,18
Licorice: The immunostimulant properties of licorice (in the active component glycyrrhizin) may negate effects of cyclosporine.3
St. John's Wort: Cases of transplant rejection have been reported with concomitant use with cyclosporine and St. John's wort. The mechanism for this interaction is proposed to be induction of CYP enzymes.24
RESEARCHING DRUG INTERACTIONS AND ADVERSE EVENTS
When drug interactions or adverse
effects are suspected with CAM therapy, pharmacists need a frontline reference
available to research these events so that they may properly direct their
patients. There are several reference books available on this topic; however,
they are not updated as frequently as primary resources.
The Natural Medicines Comprehensive Database is updated continually online and annually in personal digital assistant as well as textbook formats.27 It is an up-to-date, referenced, clinically useful resource that includes information about potential uses, safety, effectiveness, adverse effects, drug-herb, herb-herb, herb-disease state, and herb-lab interactions, as well as dosing and other pertinent sections. It is one of the few references that attempts to unveil the myriad ingredients that are often found within one brand name. For example, a certain product labeled as green tea contains multiple additional herbs and supplements, despite its misleading brand name. It may be that one of the other ingredients "hiding" in the preparation is culprit to the adverse event or interaction that the patient experienced. For pharmacists with limited available library resources who are seeking one reference to include in their pharmacy, this database may offer the most current and comprehensive information about herbal CAM.
DISCUSSING HERBAL CAM THERAPY WITH PATIENTS
Nondisclosure of herb use to
health care practitioners makes managing safety concerns a difficult task. It
is possible that in the monitoring of prescription cardiovascular drug
therapy, a benefit is attributed to the prescription drug when in reality an
herbal preparation may be, at least in part, the reason for the therapeutic
response. Conversely, when herbals are used in addition to prescription
medications, the expected therapeutic effects of prescription medication may
be negated by use of the CAM. These issues make it resoundingly clear that
health care practitioners, including pharmacists, need to become more aware
and adept at handling the issues that arise when CAM therapies are used in
addition to allopathic treatments. Discussing possible drug interaction safety
concerns with the patient in a manner that captures the patient's confidence
and trust will open the door to communication about this subject. Discussing
the patient's belief system surrounding CAM in a nonjudgmental manner is
important. For most pharmacists who are well aware of legal issues pertaining
to the practice of pharmacy, it is often difficult to recommend
non–FDA-approved therapies with little or no data to support their use;
therein lies an ethical dilemma that may at times interfere with the
patient-practitioner relationship.
Ultimately, however, it is the patient who should be in charge of his or her own health decisions after the health care team has informed the patient of the known and unknown parameters surrounding the therapy in question. Pharmacists and other health care practitioners must help patients to carefully balance benefits versus risks of using a particular CAM therapy. Some herbal CAM therapies have demonstrated efficacy and safety data; therefore, practitioners may feel compelled to recommend these treatments. In addition, disclosing to patients the issue of nonapproval by the FDA and marketing practice barriers may help to steer the patient away from experiencing adverse reactions. By using available data and recognizing seals of approval and reputable manufacturers, pharmacists and health care practitioners can make an effort to instruct patients on how to safely use herbal preparations of their choice.
CONCLUSION
Although high use of and
nondisclosure of herbal CAM make it difficult for health care practitioners to
ensure safety for their patients, there are several steps that practitioners
can take to minimize the potential for adverse outcomes. First, learn to
discuss CAM therapy with patients in order to obtain a complete medication
history. Furthermore, pharmacists and health care practitioners should arm
themselves with data. To be knowledgeable about the potential and reported
drug-herb interactions is vital in recognizing the culprit to an adverse drug
event. In addition, health care professionals should encourage patients to use
only one pharmacy, centralizing the patients' records and creating a clear
line of communication among the various prescribers that cardiac patients may
see. Finally, diligently report suspected interactions and adverse events so
that others can learn new safety information about herbals being used by their
patients. By working together with patients, prescribers, and the FDA,
pharmacists can truly help their patients who use CAM and prescription drugs
concomitantly to make balanced health care decisions.
REFERENCES
1. Eisenberg DM, Davis RB, Ettner
SL, et al. Trends in alternative medicine use in the United States, 1990-1997:
results of a follow-up national survey. JAMA. 1998;280:1569-1575.
2. Barraco D, Valencia G, Riba AL, et
al. Complementary and alternative medicine (CAM) use patterns and disclosure
to physicians in acute coronary syndromes patients. Complement Ther Med
. 2005;13:34-40.
3. Miller LG. Herbal medicinals:
selected clinical considerations focusing on known or potential drug-herb
interactions. Arch Intern Med. 1998;158:2200-2211.
4. Saper RB, Kales SN, Paquin J, et
al. Heavy metal content of ayurvedic herbal medicine products. JAMA.
2004;292:2868-2873.
5. Lau KK, Lai CK, Chan AW. Phenytoin
poisoning after using Chinese proprietary medicines. Hum Exp Toxicol.
2000;19:385-386.
6. Krochmal R, Hardy M, Bowerman S,
et al. Phytochemical assays of commercial botanical dietary supplements.
Evid Based Complement Alternat Med. 2004;1:305-313.
7. Harkey MR, Henderson GL, Gershwin
ME, et al. Variability in commercial ginseng products: an analysis of 25
preparations. Am J Clin Nutr. 2001;73:1101-1106.
8. Hathcock J. Dietary supplements:
how they are used and regulated. J Nutr. 2001;131:1114S-1117S.
9. 2006 Physicians' Desk Reference
(PDR). 60th ed. Montvale, NJ: Thomson PDR; 2005.
10. Dietary supplements: Background
information. National Institutes of Health Office of Dietary Supplements Web
site. Available at: ods.od.nih.gov/factsheets/DietarySupplements.asp. Accessed
January 29, 2006.
11. USP-verified dietary supplements.
United States Pharmacopeia Web site. Available at:
www.usp.org/USPVerified/dietarySupplements. Accessed January 29, 2006.
12. ConsumerLab Web site. Available
at: www.consumerlab.com. Accessed January 29, 2006.
13. Mashour NH, Lin GI, Frishman WH.
Herbal medicine for the treatment of cardiovascular disease: clinical
considerations. Arch Intern Med. 1998;158:2225-2234.
14. Villegas JF, Barabe DN, Stein RA,
Lazar E. Adverse effects of herbal treatment of cardiovascular disease: what
the physician must know. Heart Dis. 2001;3:169-175.
15. Bui LT, Nguyen DT, Ambrose PJ.
Blood pressure and heart rate effects following a single dose of bitter
orange. Ann Pharmacother. 2006;40:53-57.
16. Aggarwal A, Ades PA. Interactions
of herbal remedies with prescription cardiovascular medications. Coron
Artery Dis. 2001;12:581-584.
17. DerMarderosian A, Liberti L,
Beutler JA, et al. The Review of Natural Products. 4th ed. Lippincott
Williams and Wilkins; 2005.
18. Davidson P, Hancock K, Leung D,
et al. Traditional Chinese medicine and heart disease: what does Western
medicine and nursing science know about it? Eur J Cardiovasc Nurs.
2003;2:171-181.
19. Neafsey PJ. Self-medication
practices that alter the efficacy of selected cardiac medications. Home
Healthc Nurse2004;22:88-98.
20. Mansoor GA. Herbs and alternative
therapies in the hypertension clinic. Am J Hypertens. 2001;14:971-975.
21. Stewart PM, Wallace AM, Valentino
R, et al. Mineralocorticoid activity of liquorice: 11-beta-hydroxysteroid
dehydrogenase deficiency comes of age. Lancet. 1987;2:821-824.
22. Yu DK. The contribution of
P-glycoprotein to pharmacokinetic drug-drug interactions. J Clin Pharmacol
. 1999;39:1203-1211.
23. Heck AM, DeWitt BA, Lukes AL.
Potential interactions between alternative therapies and warfarin. Am J
Health Syst Pharm. 2000;57:1221-1227.
24. Piscitelli SC, Burstein AH,
Chaitt D, et al. Indinavir concentrations and St. John's wort. Lancet
. 2000;355:547-548.
25. McRae S. Elevated serum digoxin
levels in a patient taking digoxin and Siberian ginseng. CMAJ.
1996;155:293-295.
26. Chong PH, Seeger JD, Franklin C.
Clinically relevant differences between the statins: implications for
therapeutic selection. Am J Med. 2001;111:390-400.
27. Therapeutic Research Faculty.
Natural Medicines Comprehensive Database Web site. Available at:
www.naturaldatabase.com. Accessed July 25, 2006.
To comment on this article, contact editor@uspharmacist.com.






