US Pharm. 2014;39(1):35-38.
ABSTRACT: Guillain-Barré syndrome is a heterogeneous condition with several variant forms that is the most common cause of acute flaccid paralysis in both children and adults. It is estimated that the condition affects about 1 to 3 individuals per 100,000 persons. Patients typically complain of increasing pain and weakness in the limbs associated with tingling dysesthesias in the extremities. The majority of patients with Guillain-Barré have been diagnosed with an infection in the 3 weeks prior to the development of symptoms. Other events that have been linked to the disorder include vaccinations, surgery, and certain drugs. Therapy includes immunotherapy to accelerate the recovery process as well as specific therapies for individual patients such as pain relief.
Guillain-Barré syndrome (GBS) is a heterogeneous condition associated with immune-mediated, reactive, self-limiting peripheral neuropathies.1,2 It represents at least five different entities that are associated with an increased concentration of cerebrospinal fluid protein but a normal cell count and manifest as systemic motor paralysis.1,2
Three of these forms predominantly affect the motor system: acute motor axonal neuropathy (AMAN), acute motor-sensory axonal neuropathy (AMSAN), and acute inflammatory demyelinating polyradiculoneuropathy (AIDP).1 The other two variants are Miller Fisher syndrome and acute pandysautonomic neuropathy.1
GBS is the leading cause of flaccid paralysis in Western countries. It is estimated that direct healthcare costs of patients in the United States alone amount to $110,000 per person, and costs due to a loss in productivity per patient run close to $360,000 annually.2,3
The disorder, triggered by a preceding bacterial or viral infection, causes respiratory failure requiring mechanical ventilation in approximately 25% of cases.2 Mortality rates in patients requiring mechanical ventilation are as high as 20%, with persistent disability and persistent fatigue in 20% and 67% of patients, respectively.4
Epidemiology
Since there is no reference test that would allow for a positive confirmation of the diagnosis of GBS, it has been difficult to establish accurate epidemiologic data. GBS affects between 1 to 3 individuals per 100,000 persons, with males being 1.5 times more likely to be affected.5 The condition has been reported worldwide in all age groups, with the incidence increasing linearly with age.2,5 The peak incidences occur at late adolescence and in the elderly. It is thought that the bimodal peaks are possibly related to the increased risk of cytomegalovirus and Campylobacter jejuni in the former group and failing immune suppressor mechanisms in the latter population. The annual incidence in patients over age 70 years increases to 8.6 in 100,000 people. Pregnant women are at a lower risk of developing GBS; however, their risk increases immediately after delivery.2
Symptoms
GBS is characterized by rapidly evolving symmetrical limb weakness that accompanies tingling dysesthesias in the extremities.2 The weakness is more prominent in proximal muscles, with the lower limbs being more affected than the upper limbs. The paresthesias spread proximally, but rarely pass the wrists and ankles. The patient’s eye movements, swallowing movements, facial muscles, and airway maintenance may be disrupted if his or her cranial nerve is affected.2 The symptoms of the syndrome can be divided into three phases6:
1.The progressive phase, which lasts a few days to 4 weeks
2. The plateau phase, which consists of persistent symptoms and lasts for a few days or weeks
3. The improvement phase, when recovery takes place.
About half of patients with GBS complain of severe pain that is experienced with the slightest of movements. The most common sites of pain are the shoulder, girdle, back, and posterior thighs.7,8 The pain can be neuropathic as well as nociceptive in origin.9
Etiology and Pathogenesis
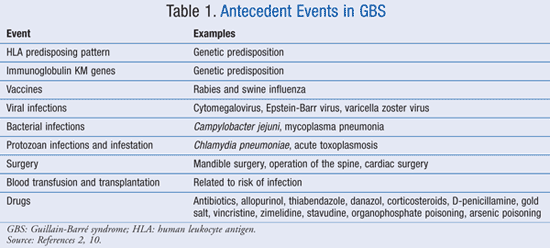
Antecedent events are implicated in the pathogenesis of GBS. TABLE 1 lists some of the identified events that lead to GBS.2,10
More than 60% of patients who are diagnosed with GBS have been diagnosed with an infection in the 3 weeks prior to the onset of weakness. The most common antecedent symptoms have been fever, cough, sore throat, and nasal discharge.5
Diagnosis
GBS is a generalized peripheral disorder that can be confused with several other conditions.7,11 The diagnosis is based upon the typical clinical features, an electrodiagnostic examination, and examination of the cerebrospinal fluid. It is useful to determine the specific subtype that the patient is suffering from since the axonal forms (AMAN and AMSAN) tend to have a poorer prognosis.2,12-16
The only clinical feature that is required for the diagnosis of GBS is progressive weakness in both arms and both legs.2 The progression of the typical symptoms over days to 4 weeks, the relative symmetry of the symptoms, the presence of mild sensory symptoms, and symptoms indicative of cranial nerve involvement and/or autonomic dysfunction strongly support the diagnosis of GBS.2
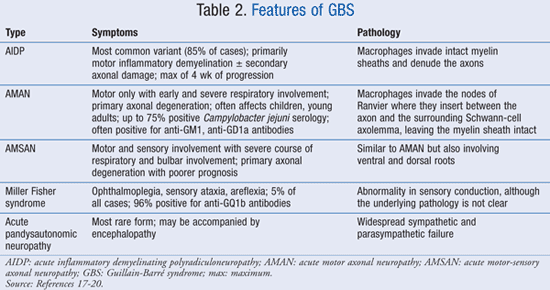
If a diagnosis of botulism, myasthenia, poliomyelitis, or toxic neuropathy is made; if abnormal porphyrin metabolism is noted; if there is a history of recent diphtheria; or if a purely sensory syndrome without weakness is observed, then GBS can possibly be ruled out.2 There are distinct features of the various types of GBS; these are listed in TABLE 2.17-20
Treatment
The overall treatment is the same for all GBS variants. Since the disease can be fatal, optimal care is provided in a hospital setting with intensive care facilities. Excellent multidisciplinary care involving supportive care as well as specific therapy is needed to manage the disease.20
Immunotherapy: Immunotherapy has been shown to accelerate recovery in patients with GBS, particularly when initiated early after motor symptoms appear. However, it is unnecessary in mild cases where no motor symptoms are exhibited. There are two forms of immunotherapy indicated for specific therapy of GBS, plasma exchange and intravenous immunoglobulin G (IVIG).1
Plasma exchange is a process that removes or dilutes the circulating immune factors implicated in the pathogenesis of GBS.5 This procedure has been shown to reduce the need for mechanical ventilation and hospitalization time by hastening recovery in nonambulant patients who seek treatment within 4 weeks of the onset of neuropathic symptoms. The maximal benefit therapy is seen if it is initiated within the first 2 weeks of onset. The usual regimen of plasma exchange is 5 times over 2 weeks, with a total exchange of about 5 plasma volumes. The disadvantages of plasma exchange include rare complications, such as sepsis. Furthermore, the use of fresh frozen plasma is associated with the risk of acquiring viral infections such as HIV.5
IVIG, which is easier to administer than plasmapheresis, is associated with fewer complications and is more comfortable for the patient.5,21 It is recommended for patients who cannot ambulate without assistance within 2 or 4 weeks of neuropathic symptom onset.4,22 The recommended dose is 0.4 g/kg per body weight daily for 5 consecutive days.5 As an added advantage, the patient’s CD8+ T-cell function is enhanced by an unknown function by the use of IVIG.23
Patients on IVIG may develop self-limiting, influenza-like symptoms including fever, myalgia, headache, nausea, and vomiting.1,23 Other side effects include aseptic meningitis, neutropenia, and hypertension. Caution must be used when administering IVIG to patients with congestive heart failure and renal insufficiency, and its use is contraindicated in those patients with a previous history of anaphylaxis to IVIG. The risk of serious hepatitis C infection transmission has been reduced significantly, following changes in preparation and purification.23
Corticosteroids: While oral corticosteroids and IV methylprednisolone were once believed to be useful in the treatment of GBS due to their immune-mediated inflammatory mechanism, they are no longer used because they do not seem to offer any benefit over immunotherapy. Furthermore, there seems to be no added advantage of adding corticosteroids to an IVIG regimen.4
Mechanical Ventilation
Even though immunotherapy has almost halved the duration of mechanical ventilation, about 25% of all patients with GBS demonstrate respiratory failure requiring ICU admission and invasive mechanical ventilation.20,24 Respiratory failure tends to be more likely in cases with rapid progression, bulbar palsy, upper limb involvement, and autonomic dysfunction.20 Endotracheal intubation and mechanical ventilation should be initiated within 24 hours of symptom onset in an ICU together with regular monitoring and measurement of vital capacity. These steps form an essential part of therapy since a positive clinical outcome largely depends upon the anticipation and management of ventilatory failure and its complications.25
Pain Management
Pain remains an undertreated but important aspect of GBS. Furthermore, patients who are immobilized and require tracheal intubation are not able to effectively express the extent of pain experienced. A pain management regimen should therefore be considered in all patients presenting with GBS.
While opioids and NSAIDs have traditionally been used in pain management plans, their host of side effects has prompted the development of safer therapeutic modalities. Opioids, while providing effective pain relief, have been linked to tolerance, dependence, respiratory depression, sedation, and constipation.9 Furthermore, they should be used with caution since there is an already present risk of ileus in patients with GBS.9,26 On the other hand, NSAIDs can lead to ulceration, bleeding, platelet dysfunction, and renal and hepatic failure.9
Anticonvulsants are frequently used in the management of neuropathic pain associated with GBS. Small trials have shown a positive analgesic effect with gabapentin or carbamazepine for pain management in GBS.27 The typical dosages used for pain management are 900 to 3,600 mg of gabapentin or 100 to 1,200 mg of carbamazepine administered as three divided doses.28
Physical therapy involving gentle massage, passive range of motion exercises, and frequent position changes may provide adjuvant relief in some patients. This may be integrated together with a rehabilitation program including occupational and physical therapy to overcome the persistent fatigue that is experienced due to the loss of axons.6,20
Cardiovascular Complications
About two-thirds of patients with GBS experience cardiovascular complications and need to be managed appropriately. These changes, attributable to autonomic neuropathy, include heart rhythm abnormalities, blood pressure variability (both hypo- and hypertension), myocardial involvement, acute coronary syndromes, and electrocardiographic changes.29
Patients with severe disease should be monitored for cardiac arrhythmia. Nonambulant adult patients are at a risk of venous thromboembolism, usually occurring between 1 to 10 weeks following the onset of symptoms.27 Subcutaneous low-molecular-weight heparin (LMWH) and graduated compression stockings may be initiated as prophylactic therapy against deep venous thrombosis.20
Conclusion
Future research needs to be focused on developing accurate diagnostic methods as well as protocols to identify at-risk patients. The role of immunotherapy in patients with GBS needs to be fully established, and effective therapies are yet to be developed.4 IV eculizumab, interferon beta, and mycophenolate mofetil are all currently undergoing investigation for use in the management of GBS.27 Other areas of research include investigating ventilator support measures and management of fatigue.4 Pharmacists have an important role to play in providing guidance on the correct use of existing therapies as well as supportive measures.
REFERENCES
1. Lindenbaum Y, Kissel JT, Mendell JR. Treatment
approaches for Guillain-Barré syndrome and chronic inflammatory
demyelinating polyradiculoneuropathy. Neurol Clin. 2001;19:187-204.
2. Hahn AF. Guillain-Barré syndrome. Lancet. 1998;2:352:635-641.
3. Buzby JC, Allos B, Roberts T. Annual costs of Guillain-Barré syndrome in the United States. Ann Neurol. 1995;38:348.
4. Hughes RA, Wijdicks EF, Barohn R, et al. Practice
parameter: Immuno-therapy for Guillain-Barré syndrome: report of the
Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61:736-740.
5. van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 2008;7:939-950.
6. Newswanger DL, Warren CR. Guillain-Barré syndrome. Am Fam Physician. 2004;69:2405-2410.
7. Ropper AH. The Guillain-Barré syndrome. N Engl J Med. 1992;326:1130-1136.
8. Ropper AH, Shahani BT. Pain in Guillain-Barré syndrome. Arch Neurol. 1984;41:511-514.
9. Gabapentin for the treatment of pain in Guillain-Barré syndrome: double-blinded, placebo-controlled, crossover study. Anesth Analg. 2002;95:1719-1723.
10. Ianello S. Guillain-Barre Syndrome: Pathological, Clinical and Therapeutical Aspects. Hauppauge, NY: Nova Science Publishers Inc; 2005.
11. Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27(suppl):S21-S24.
12. McKhann GM, Cornblath DR, Griffin JW, et al. Acute
motor axonal neuropathy: a frequent cause of acute flaccid paralysis in
China. Ann Neurol. 1993;33:333-342.
13. Ho TW, Li CY, Cornblath DR, et al. Patterns of recovery in the Guillain-Barré syndromes. Neurology. 1997;48:695-700.
14. Griffin JW, Li CY, Ho TW, et al. Pathology of the motor-sensory axonal Guillain-Barré syndrome. Ann Neurol. 1996;39:17-28.
15. Mori M, Kuwabara S, Fukutake T, et al. Clinical features and prognosis of Miller Fisher syndrome. Neurology. 2001;56:1104-1116.
16. Zochodne DW. Autonomic involvement in Guillain-Barré syndrome: a review. Muscle Nerve. 1994;17:1145-1155.
17. Prineas JW. Pathology of the Guillain-Barré syndrome. Ann Neurol. 1981;9(suppl):6-19.
18. Lampert PW. Electron microscopic studies on ordinary and hyperacute experimental allergic encephalomyelitis. Acta Neuropathol. 1967;9:99-126.
19. Pithadia AB, Kakadia N. Guillain-Barré syndrome. Pharmacol Rep. 2010;62:220-232.
20. Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366:1653-1366.
21. van der Meche FG, Schmitz PI. A randomized trial
comparing intravenous immune globulin and plasma exchange in
Guillain-Barré syndrome. Dutch Guillain-Barré Study Group. N Engl J Med. 1992;326:1123-1129.
22. Randomised trial of plasma exchange, intravenous
immunoglobulin, and combined treatments in Guillain-Barré syndrome.
Plasma Exchange/Sandoglobulin Guillain-Barré Trial Group. Lancet. 1997;349:225-230.
23. Sater RA, Rostami A. Treatment of Guillain-Barré syndrome with intravenous immunoglobulin. Neurology. 1998;51(suppl 5):S9-S15.
24. Orlikowski D, Prigent H, Sharshar T, et al. Respiratory dysfunction in Guillain-Barré syndrome. Neurocrit Care. 2004;1:415-422.
25. Teitelbaum JS, Borel CO. Respiratory dysfunction in Guillain-Barré syndrome. Clin Chest Med. 1994;15:705-714.
26. Tripathi M, Kaushik S. Carbamazepine for pain management in Guillain-Barré syndrome patients in the intensive care unit. Crit Care Med. 2000;28:655-658.
27. White-McCrimmon RY. Emergent management of Guillain-Barre syndrome. Medscape. http://emedicine.medscape.com/article/792008-overview#aw2aab6b8. Accessed September 20, 2013.
28. Pandey CK, Raza M, Tripathi M, et al. The comparative
evaluation of gabapentin and carbamazepine for pain management in
Guillain-Barré syndrome patients in the intensive care unit. Anesth Analg. 2005;101:220-225.
29. Mukerji S. Cardiovascular complications of the Guillain-Barré syndrome. Am J Cardiol. 2009;104:1452-1455.
To comment on this article, contact rdavidson@uspharmacist.com.





