US Pharm. 2016;41(1):40-44.
ABSTRACT: Narcolepsy, a complex and often debilitating neurologic disease, is characterized by cataplexy, sleep paralysis, and hypnagogic and/or hypnopompic hallucinations. Several treatments are available, such as amphetamines, modafinil, and sodium oxybate, but current therapy is limited to symptom management and does not affect the underlying process. Recognition and a better understanding of the role of autoimmunity in type 1 narcolepsy have provided a means for early diagnosis, thus creating the potential for new interventions, such as hypocretin replacement and IV immunoglobulin therapy. However, larger-scale studies need to be conducted in order to prove the efficacy of these therapies.
Narcolepsy is a complex and often debilitating neurologic disease characterized by excessive daytime sleepiness, uncontrollable sleep attacks, hypnagogic (drowsiness preceding sleep) and hypnopompic (semiconsciousness preceding waking) hallucinations, and sleep paralysis. In many cases, cataplexy (sudden, brief, episodic loss of muscle tone) occurs. Narcolepsy affects one in every 1,000 to 2,000 Americans and is slightly more prevalent among men.1 This disorder can interfere with the ability to independently perform normal daily activities such as studying, driving, and working. Symptoms usually manifest within the second decade of life and increase in severity during the third and fourth decades.1
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, divides primary hypersomnolence syndromes into three types: narcolepsy caused by hypocretin (also known as orexin) deficiency, Kleine-Levin syndrome, and syndromes with hypersomnolence unexplained by hypocretin abnormalities (generally without cataplexy).2 Kleine-Levin syndrome, a rare disorder that typically affects adolescent males, is characterized by recurring bouts of excessive sleep separated by weeks or months, along with altered behavior.3,4 The International Classification of Sleep Disorders, Third Edition, classifies narcolepsy as type 1 or type 2. Type 1 narcolepsy is differentiated from type 2 by the presence of cataplexy and/or hypocretin deficiency (<110 pg/mL) in the cerebrospinal fluid (CSF).5 Since narcoleptic patients can have deficiencies in hypocretin-producing neurons, an autoimmune process may be responsible for the destruction of hypocretin-producing cells.
Etiology and Pathophysiology
In 1999, a link was discovered between abnormalities in an orexin gene and narcolepsy.6 Abnormal human leukocyte antigen (HLA) haplotypes DRB1*15 and DQB1*0602 have since been identified in approximately 90% of narcolepsy patients, but they are present in only 12% to 25% of the general population.7,8 The incidence of these HLA haplotypes in patients with hypocretin-deficient narcolepsy indicates that autoimmunity may play a significant role in type 1 narcolepsy; however, symptoms usually manifest in response to an environmental trigger.
Narcolepsy is related to rapid eye movement (REM) sleep, which normally is preceded by approximately 90 minutes of non-REM sleep. Narcoleptic patients enter REM sleep immediately upon falling asleep, both at night and during brief sleep attacks throughout the day.7
Symptoms
Excessive daytime sleepiness (including persistent drowsiness and sudden uncontrollable sleep attacks), hypnagogic hallucinations, sleep paralysis, and cataplexy are considered hallmarks of narcolepsy and are known as the narcolepsy tetrad.9 Overwhelming fatigue and disturbed nocturnal sleep, which are often the first symptoms to manifest, may precede other symptoms by months or even years.9 Sleep attacks can happen at any time and without warning. Hypnagogic hallucinations occur at the beginning of sleep and may be auditory or visual; hypnopompic hallucinations occur upon awakening. Sleep paralysis, a phenomenon in which the patient is rendered immobile while fully conscious, may also occur upon going to sleep or awakening, but has a greater association with hypnopompia.10 Recurring episodes of cataplexy, sleep paralysis, and sleep-associated hallucinations differentiate narcolepsy from other sleep disorders. In extreme cases, loss of muscle tone can result in falls. These falls are frequently misidentified as seizures, contributing to further delays in appropriate diagnosis.
Diagnosis
Cataplexy, which is often triggered by strong emotions such as anger or surprise, is highly specific to narcolepsy disorders, occurring in about 70% of patients.1,11 When appropriately identified, cataplexy can play an important role in the early identification and subsequent diagnosis of type 1 narcolepsy.7 When narcolepsy is suspected, a positive diagnosis of type 1 narcolepsy can be made by testing for HLA-DQ6/DQB1 haplotypes and hypocretin concentrations in CSF. Traditionally, diagnoses have been made by elimination, starting with a polysomnogram and following with a multiple sleep latency test (MSLT) the next day.7 Polysomnograms utilize ECG, video monitoring, and respiratory parameters to assess REM sleep cycles. During an MSLT, the patient is prompted to take four to five naps, during which sleep latency and time until onset of REM sleep are monitored. Because there is no definitive positive test for type 2 narcolepsy, diagnosis is much more difficult and is often made by excluding other sleep disorders.
Treatment
Because the majority of hypocretin-producing cells have been destroyed by the time type 1 narcolepsy is diagnosed, lifelong therapy typically is necessary.12 Treatment is currently limited to symptomatic management through lifestyle modifications and pharmacotherapy. Medications for treating narcolepsy include dextroamphetamine, amphetamine, dextroamphetamine/amphetamine, methylphenidate, modafinil, armodafinil, and sodium oxybate.
Amphetamines are stimulants that have a direct effect on alpha- and beta-adrenergic receptors and enable the release of norepinephrine and dopamine from adrenergic neurons. This class of medications stimulates the central nervous system (CNS) by interacting with neurons in the cerebral cortex and reticular activating system. At high doses, amphetamines can inhibit monoamine oxidase (MAO). Amphetamines are contraindicated in patients with advanced arteriosclerosis, cardiovascular (CV) disease, drug abuse, glaucoma, moderate-to-severe hypertension, hyperthyroidism, or agitation, as well as within 14 days of MAO inhibitor use. In addition, all amphetamines carry a black box warning for abuse, dependence, CV damage, and sudden death.13 The American Academy of Sleep Medicine reports that amphetamines are effective for the treatment of daytime sleepiness due to narcolepsy, but there is limited information on the benefit-to-risk ratio. Therefore, other options may be preferred.14 The following sections convey more medication-specific information. For all drugs discussed below, see TABLE 1 for formulations, dosing, and adverse effects (AEs).
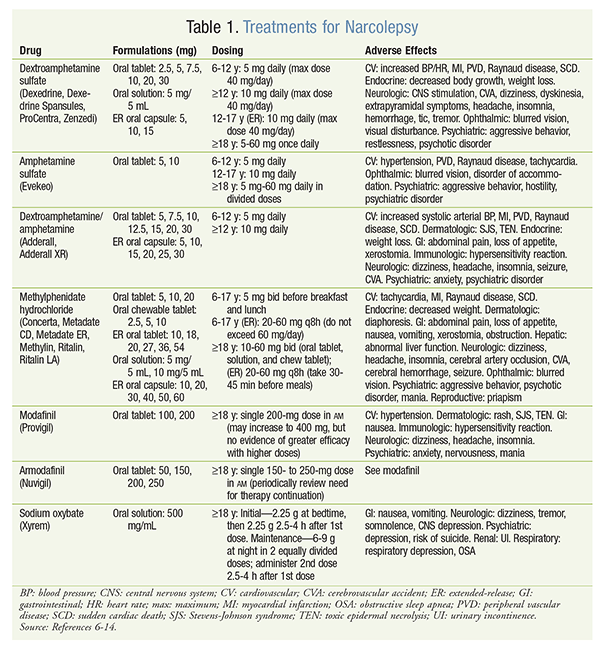
Dextroamphetamine Sulfate: Dextroamphetamine is a CNS stimulant FDA-approved for the treatment of narcolepsy and attention-deficit/hyperactivity disorder (ADHD).13 This medication is the d-isomer of amphetamine. Amphetamine d-isomers have been shown to be more active than l-isomers, and they are more specific for dopaminergic neurons than for neurons of norepinephrine and epinephrine.15
Pharmacokinetics—Oral tablets take 3 hours to reach Cmax, whereas extended-release (ER) capsules can take 8 hours. Food does not have an effect on the bioavailability of sustained-release (SR) capsules. Dextroamphetamine is metabolized to five metabolites: hippuric acid, benzoic acid, norephedrine, 4-hydroxy-norephedrine, and benzyl methyl ketone. Excretion occurs via the kidneys, and the average half-life of both the oral tablets and the ER capsules is approximately 12 hours.16
Amphetamine Sulfate: This relatively new agent is FDA-approved for the treatment of narcolepsy in patients aged 6 years and older; it is also approved for ADHD treatment and as an adjunct for the treatment of obesity. It is a noncatecholamine sympathomimetic amine with stimulatory CNS activity. Actions include systolic and diastolic elevation of blood pressure, weak bronchodilation, and stimulant respiratory action.17
Pharmacokinetics—The onset of response for amphetamine sulfate is 1 to 3 hours; the duration of action is up to 10 hours. Amphetamine sulfate is 20% protein bound, and CSF levels account for 80% of plasma levels. The drug is metabolized in the liver to form five metabolites: hippuric acid, benzoic acid, 4-hydroxyamphetamine norephedrine, 4-hydroxynorephedrine, and benzyl methyl ketone. Excretion occurs via the kidneys and depends on pH level, so the drug has a half-life of 7 to 34 hours.17
Dextroamphetamine/Amphetamine: This combination drug is FDA-approved for the treatment of ADHD and for narcolepsy in patients older than 3 years. Its expected effects and AEs are similar to those for dextroamphetamine sulfate and amphetamine sulfate. The ER form of dextroamphetamine/amphetamine is not typically used for narcolepsy since it is approved only for ADHD.18 The immediate-release (IR) form is approved for both narcolepsy and ADHD.19
Pharmacokinetics—This medication contains d- and l-isomers in a 3-to-1 ratio. The Tmax for the IR formulation is approximately 3 hours. Dextroamphetamine/amphetamine is metabolized in the liver to produce two active metabolites, 4-hydroxy-amphetamine and norephedrine. It is excreted renally, and the extent of excretion depends on urine pH and flow. Also, 50% is recovered as alpha-hydroxy-amphetamine derivatives. Since dextroamphetamine/amphetamine contains a combination of amphetamine isomers, the half-life varies by isomer. The elimination half-life of dextroamphetamine is shorter than the half-life of levoamphetamine, which is contained in amphetamine sulfate (9.77-11 hours vs. 11.5-13.8 hours).19
Methylphenidate Hydrochloride: Methylphenidate is a CNS agent that is approved for ADHD and for narcolepsy in patients aged 6 years and older. It acts on the brainstem arousal system and the cortex and is believed to exert its therapeutic effect by blocking the reuptake of norepinephrine and dopamine into the presynaptic neuron. Being an amphetamine-related drug, methylphenidate has AEs similar to those of dextroamphetamine and amphetamine. Methylphenidate carries a black box warning for its risk of dependence, abuse, and behavior changes.20
Pharmacokinetics—The absorption of methylphenidate depends on the formulation used. The Tmax is 1 to 2 hours for the IR tablets, 4.7 hours for the SR tablets, and 2 to 5 hours for the suspension. The ER capsules have two peaks: one after 1.5 to 3 hours and the second after 4.5 to 6.6 hours.8 Methylphenidate is only 10% to 33% protein bound, and 78% to 97% is excreted renally. Metabolism occurs rapidly by nonmicrosomal hydrolytic esterases in the liver and other tissue. The elimination half-life also depends on the drug formulation. The elimination half-life ranges from 2.5 to 3.5 hours for IR tablets, 2.5 to 6.8 hours for ER capsules, and 3.5 hours for ER tablets.20
Modafinil and Armodafinil: Both agents are FDA-approved for the treatment of narcolepsy in adults. Armodafinil is the R-enantiomer of modafinil. The exact mechanism by which these drugs exert their therapeutic effect is unknown; however, studies show that CNS activation occurs in discrete brain regions, suggesting a more specific wakefulness-promoting effect. The AE profiles for modafinil and armodafinil are relatively identical, but armodafinil has been associated with anaphylaxis reaction and angioedema.21-23
Modafinil seems to be the preferred treatment option for excessive daytime sleepiness based on its lower potential for abuse and lesser risk of AEs compared with amphetamines.24,25 Head-to-head clinical trials have not been conducted in humans to evaluate the efficacy of modafinil compared with amphetamines; however, modafinil 5 to 10 mg/kg and amphetamine 0.1 to 0.2 mg/kg were shown to be equally effective at increasing wakefulness and reducing deep sleep in controlled and narcoleptic dogs. Amphetamines were more effective than modafinil at suppressing cataplexy in canine subjects.26 Compared with armodafinil, modafinil has a longer duration of action, allowing once-daily administration.27 Modafinil is available in generic form, making it a more cost-effective option at this time.
Pharmacokinetics—A peak response to narcolepsy can take 1 to 2 months with modafinil.9 Modafinil takes 2 to 4 hours to reach peak concentrations, whereas armodafinil takes 2 hours. Both drugs are 60% protein bound and extensively metabolized by the liver.9-11 Modafinil is 80% excreted by the kidneys, and 10% is excreted unchanged in the urine. The excretion of armodafinil is similar to that of modafinil. The elimination half-life is 7.5 to 15 hours for modafinil and 15 hours for armodafinil.21-23 The R-isomer has a much longer duration of action, which causes the elimination of modafinil to occur in a biphasic manner. As a result, the mean AUC from time zero to infinity is 40% higher for armodafinil compared with modafinil. Based on armodafinil’s pharmacokinetic profile, plasma concentrations are higher in the evening compared with those resulting from modafinil use. Therefore, patients taking armodafinil may have decreased drowsiness and somnolence throughout the day compared with those taking modafinil.27
Sodium Oxybate: This CNS depressant is FDA-approved for the treatment of narcolepsy in adults. Safety and efficacy have not been established in pediatric patients. Sodium oxybate differs from the other drugs mentioned in that it is a designated Risk Evaluation and Mitigation Strategy (REMS) agent and is available only through the restricted-distribution Xyrem Success Program. Sodium oxybate is thought to exert its therapeutic effect through actions of gamma-aminobutyric acid type B at the noradrenergic, dopaminergic, and thalamocortical neurons. The drug carries a black box warning for obtundation and respiratory depression.28
Pharmacokinetics—The onset of sodium oxybate is 15 to 45 minutes, and the agent has a duration of 2 to 3 hours. It takes from 25 minutes to 1.25 hours for the drug’s peak concentration to be reached, and the drug is metabolized extensively by the liver. Sodium oxybate is largely excreted by expiration following biotransformation to carbon dioxide.29,30
Future Treatment Options
Hypocretin deficiency is a known cause of narcolepsy; therefore, it is likely that future treatment will address correction of the deficit. According to one study, one dose of hypocretin-1 administered intranasally to 14 patients reduced REM sleep duration, reduced wake-REM sleep transition during the day, and improved attention in type 1 narcoleptics.31 Larger-scale studies must be conducted in order to confirm efficacy. Another possible option to correct the hypocretin deficiency resides with the development of a nonpeptide hypocretin receptor agonist. However, such a chemical compound has not yet been successfully developed.32 Other options currently undergoing studies include hypocretin neuronal cell transplantation and gene therapy. The latter involves the delivery of a transgene via a viral vector that allows expression of hypocretin in animals and humans lacking the anatomical means to produce hypocretin. The recombinant adeno-associated virus has been used to deliver the hypocretin gene in mice, which led to increased hypocretin-1 levels and decreased cataplexy.33,34
Based on the presumption that narcolepsy is an autoimmune disorder, investigators are also studying the effects of IV immunoglobulin (IVIG) on narcolepsy symptoms. One study found that effects associated with IVIG administration in four patients, such as improvement in wakefulness and frequency of cataplexy, did not persist after a few weeks.35 In a second study, IVIG was administered to four patients within a few months of exhibiting narcolepsy symptoms. A reduced frequency and severity of cataplexy resulted, and this benefit was sustained for up to 7 months without any other medications being administered to treat narcolepsy post IVIG administration.36 A third study followed four children in whom high-dose IVIG was administered following early diagnosis of severe narcolepsy. One child showed persistent improvement in narcoleptic symptoms, supporting the evidence found in the second study.36,37 A large-scale randomized, controlled trial must be conducted, but the last two studies mentioned suggest efficacy association between early IVIG administration and efficacy.
Conclusion
Although narcolepsy is a complex and often debilitating neurologic disease, several therapies are available. However, current treatment is limited to symptom management. Amphetamine-like stimulants are often prescribed to control drowsiness and sleep attacks. Modafinil is prescribed to control persistent daytime sleepiness. The recent discovery of the gene that causes narcolepsy inspires hope for future development of therapies to better relieve symptoms of this disorder. Recognition and a better understanding of the role of autoimmunity in type 1 narcolepsy have also provided a means for early diagnosis, thus creating potential for new interventions, such as hypocretin replacement and IVIG therapy. Narcolepsy remains difficult to diagnose, but evolutions in pharmacotherapy are giving those living with the disease hope for the future.
REFERENCES
1. Dopp JM, Phillips BG. Sleep disorders. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill Education; 2014.
2. American Psychiatric Association. DSM-5 development. Accessed December 9, 2015.
3. What is Kleine Levin syndrome? www.kleinelevinsyndrome.co.uk. Accessed November 23, 2015.
4. National Institute of Neurological Disorders and Stroke. NINDS Kleine-Levin syndrome information page. www.ninds.nih.gov/disorders/kleine_levin/kleine_levin.htm. Accessed November 22, 2015.
5. Sateia MJ. International Classification of Sleep Disorders—third edition: highlights and modifications. Chest. 2014;146:1387-1394.
6. Nishino S, Ripley B, Overeem S, et al. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39-40.
7. Czeisler CA, Scammell TE, Saper CB. Sleep disorders. In: Kasper DL, Fauci AS, Hauser SL, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill Education; 2015.
8. Hayduk R, Flodman P, Spence MA, et al. HLA haplotypes, polysomnography, and pedigrees in a case series of patients with narcolepsy. Sleep. 1997;20:850-857.
9. Mitler MM, Hajdukovic R, Erman M, Koziol JA. Narcolepsy. J Clin Neurophysiol. 1990;7:93-118.
10. American Sleep Association. Hallucinations during sleep. www.sleepassociation.org/patients-general-public/hallucinations-during-sleep. Accessed November 22, 2015.
11. Panayiotopoulos CP. A Clinical Guide to Epileptic Syndromes and Their Treatment. 2nd ed. London, England: Springer Healthcare Ltd; 2010:122.
12. Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics. 2012;9:739-752.
13. Dextroamphetamine sulfate oral tablets, dextroamphetamine sulfate oral tablets. Micromedex (subscription database). Ann Arbor, MI: Truven Health Analytics Inc; 2015.
14. Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin: an American Academy of Sleep Medicine report. Sleep. 2007;30:1705-1711.
15. Kanbayashi T, Honda K, Kodama T, et al. Implication of dopaminergic mechanisms in the wake-promoting effects of amphetamine: a study of D- and L-derivatives in canine narcolepsy. Neuroscience. 2000;99:651-659.
16. Dextroamphetamine sulfate tablets product information. Hazelwood, MO: Mallinckrodt Inc; November 2014.
17. Evekeo (amphetamine sulfate) product information. Atlanta, GA: Arbor Pharmaceuticals, LLC; May 2015.
18. Adderall XR (dextroamphetamine/amphetamine) product information. Wayne, PA: Shire US Inc; November 2013.
19. Adderall (dextroamphetamine/amphetamine) product information. Pomona, NY: Barr Laboratories, Inc; March 2007.
20. Ritalin oral tablets, methylphenidate HCl oral tablets. Micromedex (subscription database). Ann Arbor, MI: Truven Health Analytics Inc; 2015.
21. DailyMed. Label: modafinil tablet. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9f3b0bd2-72a3-411f-aeec-b533373f1a97#section-1. Accessed July 16, 2015.
22. Nuvigil. www.nuvigil.com. Accessed December 10, 2015.
23. Nuvigil (armodafinil) product information. North Wales, PA: Teva Pharmaceuticals USA, Inc; May 2015.
24. Rush CR, Kelly TH, Hays LR, et al. Acute behavioral and physiological effects of modafinil in drug abusers. Behav Pharmacol. 2002;13:105-115.
25. Jasinski DR, Kovacevic-Ristanovic R. Evaluation of the abuse liability of modafinil and other drugs for excessive daytime sleepiness associated with narcolepsy. Clin Neuropharmacol. 2000;23:149-156.
26. Shelton J, Nishino S, Vaught J, et al. Comparative effects of modafinil and amphetamine on daytime sleepiness and cataplexy of narcoleptic dogs. Sleep. 1995;18:817-826.
27. Darwish M, Kirby M, Hellriegel ET, Robertson P Jr. Armodafinil and modafinil have substantially different pharmacokinetic profiles despite having the same terminal half-lives: analysis of data from three randomized, single-dose, pharmacokinetic studies. Clin Drug Investig. 2009;29:613-623.
28. What is Xyrem? www.xyrem.com/healthcare-professionals/xyrem-and-narcolepsy/#what-is-xyrem. Accessed July 16, 2015.
29. Xyrem dosing and titration. www.xyrem.com/healthcare-professionals/dosing-and-administration. Accessed July 16, 2015.
30. Xyrem (sodium oxybate) product information. (per FDA), Palo Alto, CA: Jazz Pharmaceuticals, Inc; April 2015.
31. Weinhold SL, Seeck-Hirschner M, Nowak A, et al. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res. 2014;262:8-13.
32. Wozniak DR, Quinnell TG. Unmet needs of patients with narcolepsy: perspectives on emerging treatment options. Nat Sci Sleep. 2015;7:51-61.
33. Liu M, Blanco-Centurion C, Konadhode R, et al. Orexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic mice. J Neurosci. 2011;31:6028-6040.
34. Blanco-Centurion C, Liu M, Konadhode R, et al. Effects of orexin gene transfer in the dorsolateral pons in orexin knockout mice. Sleep. 2013;36:31-40.
35. Valko PO, Khatami R, Baumann CR, Bassetti CL. No persistent effect of intravenous immunoglobulins in patients with narcolepsy with cataplexy. J Neurol. 2008;255:1900-1903.
36. Dauvilliers Y, Carlander B, Rivier F, et al. Successful management of cataplexy with intravenous immunoglobulins at narcolepsy onset. Ann Neurol. 2004;56:905-908.
37. Plazzi G, Poli F, Franceschini C, et al. Intravenous high-dose immunoglobulin treatment in recent onset childhood narcolepsy with cataplexy. J Neurol. 2008;255:1549-1554.
To comment on this article, contact rdavidson@uspharmacist.com.





