US Pharm. 2009;34(2):28-39.
This review addresses some of the contemporary issues pharmacists should keep in mind when counseling patients receiving warfarin therapy. Screening patients for consumption of certain foods, such as grapefruit, cranberry, and vitamin K-containing green leafy vegetables, may help reduce the risk of a food-drug interaction. Also, screening for lifestyle habits such as alcohol and tobacco use may help optimize care in patients taking warfarin.
Direct pharmacist-patient contact that includes taking patient medication histories and assuring adherence and compliance has been shown to improve medication efficacy.1-5 Pharmacists who counsel patients uphold safety profiles by avoiding drug misadventures such as interactions and side effects.6,7 Therefore, it is important to take the time to screen patients for potential drug interactions with certain foods and lifestyle habits.
Grapefruit
Grapefruit has the potential to interact with warfarin by inhibiting its metabolism.8 Warfarin contains two racemic mixtures, S-enantiomers and R-enantiomers.9 CYP450 isozymes 1A2 and 3A4 are the principal isozymes for metabolizing the R-enantiomer.10 Isozyme 2C9 is the principal isozyme for metabolizing the more potent S-enantiomer.11,12 Other metabolic pathways include CYP2C8, CYP2C18, and CYP2C19.9
Grapefruit has been found to inhibit the 1A2 and 3A4 isozymes, thus repressing warfarin metabolism.13-15 Literature suggests that grapefruit's components naringin, naringenin, and bergapten are responsible for this inhibition.13,16
Bartle: This case report detailed a potential interaction of grapefruit with warfarin.17 A 64-year-old man had been taking warfarin for atrial fibrillation (AF) since 1995. His international normalized ratios (INRs) were in therapeutic range (goal 2-3) 50% of the time in April 1996. His INR was 6.29 on April 11. No medical complaints, medications, or adverse effects involving bleeding were noted at the time of the supratherapeutic result. On April 1, however, the patient started consuming 1.5 L grapefruit juice daily. Warfarin was withheld for two days, then resumed at the usual regimen of 55 mg/week. Upon resumption, the patient discontinued grapefruit consumption. A week later, his INR was 1.82. This regimen resulted in 60% of the patient's INRs being in therapeutic range, with the highest INR being 3.24.
Sullivan et al: This small, open-label study examined the interaction between warfarin and grapefruit.18 Patients with dietary and medication changes in the past month, previous grapefruit consumption, previous drug-drug interaction with warfarin (cyclosporine, dihydropyridines, terbinafine), or alcohol consumption were excluded. The nine men studied were on a stable warfarin regimen (no dosage changes in the past four weeks and ≤10% fluctuation in two consecutive prothrombin-time [PT] results). Patients were 85.7% to 100% compliant with their warfarin doses. INRs (goal 2-3) were measured for nine days (baseline, day 0), during which patients consumed 8 oz. grapefruit juice (starting on day 1) three times daily for a week (TABLE 1). No significant difference in PT and INR values was found in any patient.
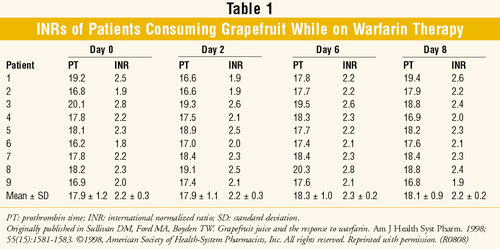
Unlike Bartle's case report, this study showed no interaction between warfarin and grapefruit. The lack of interaction may be due to the fact that the CYP enzymes inhibited by grapefruit do not affect the more potent S-enantiomer of warfarin.
Practical Application: Concurrent use of warfarin with grapefruit may increase INRs. Literature reports that CYP450 isozymes may have an influence on the pharmacodynamics and pharmacokinetics of warfarin. Therefore, it is prudent to include screening of grapefruit consumption in the anticoagulation clinic. Close monitoring may be necessary with this combination, especially if there is a big change in grapefruit consumption.
Cranberry
The potential interaction between cranberry juice and warfarin has received attention for several years. Reports suggest that consumption of large amounts of cranberry juice while on warfarin may increase patient INRs, even leading to hemorrhage in some cases.
Suvarna et al: One of the first such reported cases was of a man in his 70s who was hospitalized and later died from gastrointestinal (GI) and pericardial hemorrhages.19 The patient, on warfarin therapy, had been consuming cranberry juice for six weeks. His INR at admission was greater than 50; prior to the cranberry juice, it had been considered stable. No information about past INRs was provided.
Aston et al noted, however, that other factors may have influenced the case.20 Two weeks prior to hospitalization, the patient completed a course of cephalexin for a respiratory infection, and decreased food consumption may have led to a vitamin K deficiency that normally would counterbalance INR levels.19,20 The patient's infection also may have contributed to the outcome.
Grant: This case reported an interaction between warfarin and cranberry juice that caused high INRs, hematuria, and rectal bleeding.21 A 69-year-old man with no history of abnormal bleeding who was scheduled for sigmoid colectomy was receiving warfarin for AF. Upon admission, warfarin was discontinued, surgery was delayed, and the patient's INR was 12; prior to hospitalization, his INRs never exceeded 4. Coagulation status was checked on days 2 and 4, with an INR of 10 on both days. Vitamin K was administered, resulting in an INR of 2 within two days, and the surgery was performed. Several days after surgery and resumption of warfarin, the patient had an INR of 11 and developed hematuria and rectal bleeding. His medications were ruled out as a cause. After surgery, he received paracetamol (acetaminophen) and codeine. Acetaminophen can interact with warfarin, but the patient's supratherapeutic INR upon admission could not be explained, as he was taking only digoxin at the time.20,22,23
Two weeks before admission, upon his general practitioner's recommendation, the patient had started drinking 2 L cranberry juice daily to prevent urinary tract infection, and this practice was resumed soon after surgery. After the cranberry juice was discontinued, INRs decreased to 3, the hematuria and rectal bleeding stopped, and the patient recovered.
Rindone & Murphy: This case reported hypoprothrombinemia and bleeding that occurred with concurrent cranberry juice and warfarin.24 A 71-year-old man was admitted for hemoptysis, hematochezia, and shortness of breath for two days. Upon admission, his INR exceeded 18 (PT >120 sec) and hemoglobin (Hb) was 8.8 g/dL (baseline, 15.3 g/dL). Treatment included warfarin discontinuation, intravenous (IV) normal saline, 2 units of packed red blood cells, 1 unit of fresh-frozen plasma, and 5 mg subcutaneous (SC) vitamin K. Gatifloxacin 200 mg IV was administered every 24 hours for suspected exacerbation of chronic bronchitis. Bleeding stopped the next day, but the INR remained elevated (7), so 2.5 mg vitamin K was given SC. On day 5, the patient's INR was 2.6 and Hb was 11.5 g/dL. Upon discharge, the patient's warfarin was restarted at 14 mg/week, but later had to be increased to 18 mg/week based on two subtherapeutic INRs.
Prior to hospitalization, the patient's warfarin regimen had been stable at 18 mg/week for three months, and the INR was within goal (2-3). The only change was that, two weeks before hospital admission, the patient had begun daily consumption of 24 oz. cranberry juice for vitamin C supplementation. This practice was not restarted after his hospital stay. Aston et al pointed out that infection may have the potential to affect INR results while a patient is taking warfarin.20
Committee on Safety of Medicine: The United Kingdom's Medicines and Healthcare Products Regulatory Agency (governing body regulating the safety and efficacy of medicine and medical devices) stated in October 2004 that patients taking warfarin should avoid cranberry products unless the benefits clearly outweigh the risks.25 The U.K.'s Committee on Safety of Medicine (CSM) first advised limiting or avoiding cranberry consumption concurrently with warfarin after five cases of interactions were reported in September 2003.26 One case described an INR greater than 50 and subsequent fatality due to GI and pericardial hemorrhage in a patient who had been drinking cranberry juice for six weeks. Two cases reported supratherapeutic INRs while warfarin was taken concurrently with cranberry juice; one patient was stabilized after the warfarin dose was lowered, whereas the other patient's INR returned to therapeutic range after the juice was discontinued. Another case described an unstable INR during cranberry consumption. The final case demonstrated a decrease in INR, contradicting most published literature.
Based on these results, a second CSM publication reiterated the need to avoid cranberry products concurrently with warfarin unless the benefits outweigh the risks.27 Data were insufficient to determine a safe quantity or brand of, or difference between, cranberry juice and other cranberry products with concurrent warfarin. The CSM recommended tighter medical supervision and INR monitoring in patients consuming cranberry juice. Twelve cases with this possible interaction have been reported.
Greenblatt: A concern about the validity of the interaction between warfarin and cranberries was raised.28 This article pointed out that case reports were the only sources of a potential interaction; no randomized, double-blind, placebo-controlled study has shown an interaction. Importantly, all of the cases with an interaction involved large quantities of cranberry juice.
Practical Application: The FDA Center for Drug Evaluation and Research approved the change of the package insert for warfarin (Coumadin) on October 5, 2006. The statement recommended avoiding consumption of cranberry products while on the anticoagulant.29 Based on this, any provider managing patients who are taking warfarin should screen them for cranberry consumption and educate them about the potential interaction. Close monitoring may be warranted in patients taking warfarin and large amounts of cranberry to avoid the risk of supratherapeutic INRs. Clinical studies are needed to determine whether there is a definite interaction.
Tobacco
The pharmacodynamics of warfarin should be carefully considered in patients using tobacco products. Some studies show no difference between smokers and nonsmokers in average warfarin dose used, but changes in dose may be necessary in patients who change their smoking status.
Mitchell: This author, of the Boston Collaborative Drug Surveillance Program, reviewed data about the influence of cigarette smoking on the anticoagulant effect of warfarin.30 Information about patients on warfarin therapy was obtained from seven hospitals throughout North America. Nonsmokers were those who had never smoked or had discontinued smoking for a year; light smokers were those who smoked up to 20 cigarettes per day; and heavy smokers were those who smoked more than 20 cigarettes per day. Mean warfarin dose in nonsmokers (n = 86) was 7.46 ± 0.37 mg/day, in light smokers (n = 97) was 7.86 ± 0.35 mg/day, and in heavy smokers (n = 47) was 7.57 ± 0.50 mg/day. There was no difference in maintenance warfarin dose among groups. The report did not discuss such things as need for dosage adjustment or efficacy status, however.
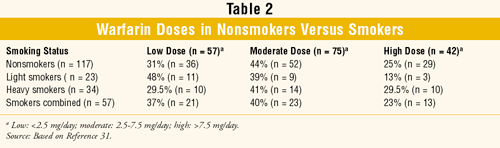
Weiner et al: This retrospective chart review also compared warfarin doses between 174 smokers and nonsmokers.31 The following inclusion criteria were used: undergoing heart-valve replacement, normal liver-function tests (LFTs) and renal-function tests, and PT 10 to 12 sec. Upon admission, each patient was screened for medications, diet, allergies, smoking status, and social habits; patients needing a medication known to interact with warfarin were excluded.
Patients were categorized as nonsmokers, light smokers, or heavy smokers.30 They were then subcategorized as taking a low, moderate, or high daily dose of warfarin (TABLE 2). Comparison also was made between combined smokers (light and heavy smokers) and nonsmokers. Regimens were designed to maintain PT at twice the control level. No difference in daily maintenance warfarin dose was noted between smokers and nonsmokers, and no change in smoking habits was found.
Colucci & Knapp: A contradictory study found an increase in INR after a patient quit smoking.32 After smoking cigarettes for more than 30 years, an 80-year-old man stopped his 50-pack/year habit. He had been taking warfarin for cerebral vascular accident for five years (INR target 2-3), and his dose had been 5 mg/day for the past 10 months (35 mg/wk). INRs ranged from 2.0 to 2.8. The patient denied any changes in medication, vitamin K, or alcohol consumption. Past medical history included chronic stable angina and hyperlipidemia. The patient reported compliance with his medications, which included simvastatin 20 mg/day, isosorbide mononitrate 60 mg/day, and topical mometasone furoate. Two months after smoking cessation, the patient's INR was elevated (3). His warfarin dose was reduced to half (2.5 mg) of his daily dose for two days, then increased back to 5 mg/day. Two weeks later, the patient's INR was supratherapeutic (3.7). His warfarin dose was held for one day, temporarily decreased to 2.5 mg daily for two days, and restored to 5 mg daily. After another two weeks, the patient's INR was still 3.7. This time the patient's warfarin dose was held for one day, then restarted at 30 mg/week (14% reduction). An LFT was performed and found to be within normal limits. The patient's INRs decreased to a range of 2.0 to 2.8 for the next nine months, and he remained tobacco-free.
Evans & Lewis: Another case supported an increase in INR after smoking cessation.33 A 58-year-old man was admitted to the hospital for bacterial meningitis. His past medical history included factor V Leiden (a hypercoagulability disorder), a positive test of lupus anticoagulant, and previous deep venous thrombosis (DVT). Seven months prior to hospitalization, he developed a DVT in his right leg. He was stabilized on warfarin 58.75 mg/week and remained in therapeutic range (INR goal 2-3) for approximately six months prior to his admission for meningitis. He was taking no other medications.
The patient's INR was 2 upon admission, and warfarin was withheld. Heparin drip was administered for the first 24 hours, then switched to dalteparin. Vancomycin 1 g and cefotaxime 2 g were administered IV every six hours. On day 6, oral warfarin 10 mg was administered once, after which 5 mg/day was given. The patient was discharged on day 8 with IV ceftriaxone 2 g every 12 hours for seven days and oral levofloxacin 750 mg/day for 14 days. Oral warfarin 10 mg was again given one time, but the maintenance dose was increased to 61.25 mg/week (approximately 8.75 mg/day). Dalteparin was continued as a bridge until the INR reached therapeutic range.
The patient's INR was 2.8 two days postdischarge; dalteparin was discontinued, and warfarin was reduced to the prehospitalization dose of 58.75 mg/week. The INR was 2 a week later, but one month postdischarge it was supratherapeutic (3.4). Given the potential interaction of warfarin with the antibiotics, INR was rechecked two months postdischarge and found to be 5.5. Alcohol consumption (3-5 glasses of wine/day) was ruled out as an influence. The patient consumed no alcohol for one month after discharge to avoid interactions with his antibiotics. His LFT during hospitalization was within normal limits. The patient discontinued his 39-pack/year smoking habit at admission and did not resume smoking. His overall warfarin dose had to be decreased to 43.75 mg/week (average 6.25 mg/day). This was a 23% reduction compared with his prehospitalization regimen.
Kuykendall et al: This study reported a lack of anticoagulation efficacy due to a potential interaction of warfarin and smokeless tobacco.34 A 34-year-old man had been taking warfarin intermittently for about 4.5 years, with the highest dose taken being 185 mg/week, but his INR values never exceeded 2. The patient's medical history included four myocardial infarctions and two ischemic strokes. He had no history of coagulopathy problems and his liver function was normal. His medication profile consisted of diphenhydramine for allergies as needed and minimal use of nonsteroidal anti-inflammatory drugs. He did not use illicit drugs, vitamins, or herbal products, but routinely used 2.5 cans of smokeless tobacco per day (1.2-oz. can = 34 g tobacco). Upon discontinuation of his tobacco habit, the patient's INR rose to 2.3 within six days.
The authors stated that the high vitamin K content of the smokeless tobacco caused the effect on warfarin. Each gram of tobacco contained about 50 mcg phylloquinone. This was estimated to be 10 to 30 times higher than the amount of vitamin K in foods such as broccoli and spinach.35
Practical Application: Tobacco products may have an effect on INR, especially when there are acute changes in the quantity used. Therefore, INRs should be monitored closely and warfarin adjusted as necessary after tobacco cessation.
Alcohol (Ethanol)
Alcohol is another lifestyle factor that should be closely monitored in patients taking warfarin. Acute alcohol consumption may increase the anticoagulation effect by decreasing the metabolism of warfarin; chronic alcohol intake, however, may actually decrease the anticoagulation effect by increasing the warfarin metabolism.36
Weathermon & Crabb: A mechanistic effect of alcohol was explained in a review article.36 Upon transportation to the liver via the bloodstream, alcohol is broken down by alcohol dehydrogenase into acetaldehyde by oxidation. Since the metabolite is toxic, it is broken down into acetate by two types of aldehyde dehydrogenase isozymes. In addition, alcohol can be metabolized by the CYP system, which plays an important role in drug interactions. CYP sites, which are embedded in the endoplasmic reticulum, are open to competing substrates of medications and alcohol. Warfarin is metabolized primarily by the liver. Although alcohol primarily targets CYP2E1, both CYP1A2 and CYP3A4 are significantly affected also.
A second mechanism of action may be displacement from proteins. Warfarin is 99% bound to plasma proteins.9 Drugs that affect the binding of warfarin to plasma proteins may pose a risk of serious drug interactions that may require careful monitoring. Human serum albumin (HSA) was the only plasma protein found in blood that bound to warfarin.37 Approximately 1% unbound warfarin was found to be available to sites of action and therefore produced the pharmacodynamic effect of anticoagulation.38
Ha et al: Consumption of alcohol (i.e., ethanol) may have the potential effect of displacing warfarin from HSA. This study examined the effects of an in vitro drug interaction of warfarin displaced from albumin with ethanol.39 After titration of ethanol, the reduction of fluorescence and the equilibrium of media by dialysis experiments have shown a significant displacement of warfarin from HSA compared with control, thereby making free warfarin more available for its pharmacologic effect.
O'Reilly (1): This study explored in vivo interactions between warfarin and mealtime alcohol in eight healthy men.40 Warfarin taken alone was compared with warfarin taken concurrently with 10 oz. and 20 oz. of daily mealtime wine (DMW). Subjects refrained from medication and alcohol for two months prior to the study. For 21 days, all subjects received daily warfarin alone. Fifteen mg warfarin once daily was administered for the first three to four days to reduce the one-stage PT to 40% of normal activity; the regimen was then reduced to 7.5 mg once daily by day 4 or 5 to maintain prothrombin activity within a range of 25% to 35%. Blood samples were taken daily or every other day.
After a four-week drug holiday, the experiment was repeated, with the addition of 10 oz. DMW for 21 days. Seven subjects received 5 oz. wine with lunch and again with dinner. Another course of blood samples was collected. A second four-week drug holiday ensued, followed by a 21-day trial of warfarin with 20 oz. DMW. Six subjects received 7.5 oz. wine with lunch, 7.5 oz. with dinner, and 5 oz. with a bedtime snack. Blood samples were collected.
There was no significant difference between warfarin alone and warfarin taken with 10 oz. or 20 oz. DMW. Average plasma level of warfarin alone (n = 2.9 mic/mL) was similar to levels of warfarin taken with 10 oz. and 20 oz. DMW (n = 2.9 mic/mL and n =3.1 mic/mL, respectively). Average prothrombin activity of warfarin alone (n = 33%) was similar to that of warfarin taken with 10 oz. and 20 oz. DMW (n = 33% and n = 32%, respectively). Therefore, 10 oz. and 20 oz. DMW had no significant effect on therapeutic hypothrombinemia in healthy subjects.
O'Reilly (2): Because alcohol absorption may decrease by 75% when alcohol is consumed with a meal versus the fasting state, O'Reilly conducted another study of alcohol and warfarin, this time in subjects who fasted for six hours before bedtime and consumed nothing for the next six hours.41,42 The subjects--seven healthy men aged approximately 21 to 30 years--refrained from medication and alcohol for two months before the study. For 21 days, subjects received daily warfarin alone. Fifteen mg warfarin was administered once daily for the first three to four days to reduce the one-stage PT to 40% of normal activity. After this, the regimen was reduced to 7.5 mg once daily by day 4 or 5 to maintain prothrombin activity within a range of 25% to 35%. Blood samples were taken daily or every other day. This experiment was repeated after a four-week drug holiday, this time with 296 mL wine included in the 21-day trial.
This study showed no significant difference between warfarin taken alone and taken with 296 mL wine during the fasting state in terms of one-stage prothrombin activity (both, 31%) and warfarin plasma levels (2.9 mic/mL vs. 3.0 mic/mL). Safety aspects were monitored (e.g., ecchymoses and bleeding), but no adverse events were reported.
Karlson et al: This study concerned the warfarin-alcohol interaction in patients with DVT or pulmonary embolism.43 Even though the study mainly involved the effects of single administration of a vitamin K pill and once-only consumption of broccoli or spinach on warfarin therapy, the authors conducted a side study of a single intake of 37.5 cL wine (41 g ethanol). Patients (n = 21; 10 women) had to be on a well-controlled warfarin regimen. No significant changes in prothrombin activity as determined by Thrombotest were seen. Therefore, a one-time intake of alcohol would be acceptable in patients taking warfarin. Long-term or repetitive consumption of wine was not studied, however. The authors concluded that occasional consumption of moderate amounts of wine will not significantly affect patients taking warfarin.
Kater et al: A comparison study was conducted of drug-rate clearance in alcoholic subjects versus a control group of subjects taking warfarin.44 Those with a history of ascites, jaundice, or abnormal LFTs were excluded. Subjects refrained from medication for three months prior to the study. Alcoholic subjects (n = 15) were those who had consumed more than 250 g alcohol daily for at least three months. The control group (n =11) comprised patients who had consumed less than 40 g alcohol in the past two weeks. Subjects had a prothrombin activity of 100% according to Thrombotest prior to the study. Each subject fasted and had a single dose of warfarin 40 mg observed for 96 hours. To avoid absorption influence, food intake was allowed two hours after drug administration.
Mean rate of warfarin clearance in alcoholic subjects was shorter than in controls (26.5 h ± 13.3 h vs. 41.1 h ± 19.2 h; P <.02). There was no significant between-group difference in PT prolongation, however. The accelerated rate of warfarin clearance in alcoholic subjects compared with controls may be due to either increased metabolism or increased excretion. The present experiment favored increased metabolism, since other studies have shown that alcohol consumption seems to increase metabolic activity in the liver.45-47
Havrda et al: Although other studies have demonstrated no significant effect of alcohol on warfarin therapy, this case report indicates an enhanced effect based on INR as a form of monitoring and assessment.48 A 58-year-old man who had been taking warfarin for 11 years to prevent ischemic stroke had an INR of 8 (goal 2-3) during a clinic visit. A second blood sample yielded the same supratherapeutic result. His dose was 93.75 mg/week. His INRs had been in therapeutic range (1.9-2.5; mean 2.18) for more than five months. He had no recent illnesses or any problems with bleeding or bruising; reported compliance with therapy; had no changes in vitamin K intake; used no tobacco products; and had no changes in medications, which included prescription, OTC, and herbal products. The patient's medication profile included aspirin 325 mg daily, atorvastatin 20 mg at bedtime, gemfibrozil 600 mg twice daily, ipratropium inhaler 2 puffs three times daily, isosorbide dinitrate 20 mg twice daily, levothyroxine 0.1 mg daily, metoprolol 100 mg twice daily, multivitamin daily, nitroglycerin spray as needed, and rosiglitazone 4 mg twice daily. The last medication adjusted was levothyroxine, eight months prior to the INR of 8. The patient's medical history included coronary heart disease, type 2 diabetes mellitus, gastroesophageal reflux disease, hypertension, hyperreactive airway, hypothyroidism, and ischemic stroke. Thyroid-stimulating hormone was within normal limits five months prior.
The patient admitted to drinking alcohol for its cardioprotective effect. Although he had refrained from alcohol for 35 years, he started consuming half a can of beer (5.3 g) every other day before the time of the supratherapeutic blood result.
Due to excessive anticoagulation, the patient's INR was rechecked after withholding warfarin for three days and refraining from alcohol consumption. The INR was 2, and warfarin was restarted at a lower dose (90 mg/wk). After one week, the INR was 1.9 and the patient was instructed to continue on this regimen. However, the INR was 1.5 at another follow-up 2.5 weeks later. Warfarin was titrated up to the patient's original regimen of 93.75 mg/week. His INRs were stabilized back into therapeutic range, and the patient did not restart consuming alcohol.
Practical Application: The studies comparing warfarin alone and with alcohol consumption did not find a significant difference in effect on anticoagulation. They seem to support the conclusion that it may be safe to consume small amounts of alcohol while on concurrent warfarin therapy. These studies were small, however. Only one study had patients with indications for long-term warfarin; the other studies involved short-term use in healthy subjects or subjects without indications for the anticoagulant. One of these studies utilized one-time-only consumption of alcohol. The case report, however, suggests that some patients may not tolerate alcohol with warfarin when anticoagulation status is measured by INR. Therefore, pharmacists should monitor warfarin patients for alcohol consumption and educate them about the possible interaction between ethanol and warfarin.
Conclusion
Warfarin has a narrow therapeutic index and requires careful monitoring.9 Each patient is highly individualized based on response to the anticoagulant. Warfarin has the potential to be influenced by factors both endogenous and exogenous.
The literature reviewed in this article suggests that pharmacists counsel and screen all warfarin patients for the potential interaction with grapefruit. Cranberry consumption should be assessed for the same reason. Grapefruit and cranberry may cause a safety concern resulting from the supratherapeutic effect of warfarin. Questions about tobacco use and alcohol consumption should be included in the counseling and screening process, as these substances can affect warfarin's safety and efficacy.
REFERENCES
1. Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100:428-437.
2. Chisholm MA, Vollenweider LJ, Mulloy LL, et al. Direct patient care services provided by a pharmacist on a multidisciplinary renal transplant team. Am J Health Syst Pharm. 2000;57:1599-1601.
3. Strand LM, Cipolle RJ, Morley PC, Frakes MJ. The impact of pharmaceutical care practice on the practitioner and the patient in the ambulatory practice setting: twenty-five years of experience. Curr Pharm Des. 2004;10:3987-4001.
4. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427-440.
5. Haines ST. Making residency training an expectation for pharmacists in direct patient care roles. Am J Pharm Educ. 2007;71:71.
6. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565-571.
7. De Young M. Research on the effects of pharmacist-patient communication in institutions and ambulatory care sites, 1969-1994. Am J Health Syst Pharm. 1996;53:1277-1291.
8. Runkel M, Tegtmeier M, Legrum W. Metabolic and analytical interactions of grapefruit juice and 1,2-benzophyrone (coumarin) in man. Eur J Clin Pharmacol. 1996;50:225-230.
9. Coumadin (warfarin sodium) package insert. Princeton, NJ: Bristol-Myers Squibb Co; April 2005.
10. Okey AB. Enzyme induction in the cytochrome P-450 system. Pharmacol Ther. 1990;45:241-298.
11. Rettie AE, Korzekwa KR, Kunze KL, et al. Hydroxylation of warfarin by human cDNA-expressed cytochrome P-450: a role for P-4502C9 in the etiology of (S)-warfarin-drug interactions. Chem Res Toxicol. 1992;5:54-59.
12. Eble JM, West BD, Link KP. A comparison of the isomers of warfarin. Biochem Pharmacol. 1966; 15:1003-1006.
13. Fuhr U, Klittich K, Staib AH. Inhibitory effect of grapefruit juice and its bitter principal, naringenin, on CYP1A2 dependent metabolism of caffeine in man. Br J Clin Pharmacol. 1993;35:431-436.
14. Ducharme MP, Warbasse LH, Edwards DJ. Disposition of intravenous and oral cyclosporine after administration with grapefruit juice. Clin Pharmacol Ther. 1995;57:485-491.
15. Hulisz D, Jakab J. Food-drug interactions: which ones really matter? US Pharm. 2007;32(3):93-98.
16. Ho PC, Saville DJ, Coville PF, Wanwimolruk S. Content of CYP3A4 inhibitors, naringin, naringenin and bergapten in grapefruit and grapefruit juice products. Pharm Acta Helv. 2000;74:379-385.
17. Bartle WR. Grapefruit juice might still be a factor in warfarin response. Am J Health Syst Pharm. 1999;56:676.
18. Sullivan DM, Ford MA, Boyden TW. Grapefruit juice and the response to warfarin. Am J Health Syst Pharm. 1998;55:1581-1583.
19. Suvarna R, Pirmohamed M, Henderson L. Possible interaction between warfarin and cranberry juice. BMJ. 2003;327:1454.
20. Aston JL, Lodolce AE, Shapiro NL. Interaction between warfarin and cranberry juice. Pharmacotherapy. 2006;26:1314-1319.
21. Grant P. Warfarin and cranberry juice: an interaction? J Heart Valve Dis. 2004;13:25-26.
22. Parra D, Beckey NP, Stevens GR. The effect of acetaminophen on the international normalized ratio in patients stabilized on warfarin therapy. Pharmacotherapy. 2007;27:675-683.
23. Mahé I, Betrand N, Drouet L, et al. Interaction between paracetamol and warfarin in patients: a double-blind, placebo-controlled, randomized study. Haematologica. 2006;91:1621-1627.
24. Rindone JP, Murphy TW. Warfarin-cranberry juice interaction resulting in profound hypoprothrombinemia and bleeding. Am J Ther. 2006;13:283-284.
25. Medicines and Healthcare Products Regulatory Agency. Current Safety Issues. October 1, 2004. Cranberry. www.mhra.gov.uk/Howweregulate/
26. Committee on Safety of Medicines. Interaction between warfarin and cranberry juice: new advice. Curr Prob Pharmacovigilance. 2003;29:1-10.
27. Committee on Safety of Medicines. Interaction between warfarin and cranberry juice: new advice. Curr Prob Pharmacovigilance. 2004;30:1-12.
28. Greenblatt DJ. Cranberry juice & warfarin: is there an interaction? Anti coagulation Forum.
29. Coumadin (warfarin sodium) package insert. Princeton, NJ: Bristol-Myers Squibb Co; April 2006. www.fda.gov/cder/Offices/ODS/
30. Mitchell AA. Smoking and warfarin dosage. N Engl J Med. 1972;287:1153-1154.
31. Weiner B, Faraci PA, Fayad R, Swanson L. Warfarin dosage following prosthetic valve replacement: effect of smoking history. Drug Intell Clin Pharm. 1984;18:904-906.
32. Colucci VJ, Knapp JF. Increase in international normalized ratio associated with smoking cessation. Ann Pharmacother. 2001;35:385-386.
33. Evans M, Lewis GM. Increase in international normalized ratio after smoking cessation in a patient receiving warfarin. Pharmacotherapy. 2005;25:1656-1659.
34. Kuykendall JR, Houle MD, Rhodes RS. Possible warfarin failure due to interaction with smokeless tobacco. Ann Pharmacother. 2004;38:595-597.
35. Olson R. Vitamin K. In: Shils ME, Olson JA, Shike M, eds. Modern Nutrition in Health and Disease.
36. Weathermon R, Crabb DW. Alcohol and medication interactions. Alcohol Res Health. 1999;23:40-54.
37. Wilting J, van der Giesen WF, Janssen LH, et al. The effect of albumin conformation on the binding of warfarin to human serum albumin. J Biol Chem. 1980;255:3032-3027.
38. Yacobi A, Udall JA, Levy G. Serum protein binding as a determinant of warfarin body clearance and anticoagulant effect. Clin Pharmacol Ther. 1976;19:552-558.
39. Ha CE, Petersen CE, Park DS, et al. Investigations of the effects of ethanol on warfarin binding to human serum albumin. J Biomed Sci. 2000;7:114-121.
40. O'Reilly RA. Lack of effect of mealtime wine on the hypothrombinemia of oral anticoagulants. Am J Med Sci. 1979;277:189-194.
41. Wilkinson PK, Sedman AJ, Sakmar E, et al. Fasting and nonfasting blood ethanol concentrations following repeated oral administration of ethanol to one adult male subject. J Pharmacokinet Biopharm. 1977;5:41-52. 1967;19:317-366.
42. O'Reilly RA. Lack of effect of fortified wine ingested during fasting and anticoagulant therapy. Arch Intern Med. 1981;141:458-459.
43. Karlson B, Leijd B, Hellström K. On the influence of vitamin K-rich vegetables and wine on the effectiveness of warfarin treatment. Acta Med Scand. 1986;220:347-350.
44. Kater RM, Roggin G, Tobon F, et al. Increased rate of clearance of drugs from the circulation of alcoholics. Am J Med Sci. 1969;258:35-39.
45. Rubin E, Hutterer F, Lieber CS. Ethanol increases hepatic smooth endoplasmic reticulum and drug-metabolizing enzymes. Science. 1968;159:1469-1470.
46. Conney AH. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev.
47. Rubin E, Lieber CS. Hepatic microsomal enzymes in man and rat: induction and inhibition by ethanol. Science. 1968;162:690-691.
48. Havrda DE, Mai T, Chonlahan J. Enhanced antithrombotic effect of warfarin associated with low-dose alcohol consumption. Pharmacotherapy. 2005;25:303-307.
To comment on this article, contact rdavidson@jobson.com.





