US Pharm. 2009;34(10):HS14-HS18.
The National Library of Medicine (NLM) introduced the term evidence-based medicine (EBM) as a medical subject heading to PubMed in 1997.1 It is defined as: “An approach of practicing medicine with the goal to improve and evaluate patient care. It requires the judicious integration of best research evidence with the patient’s values to make decisions about medical care. This method is to help physicians make proper diagnosis, devise best testing plan, choose best treatment and methods of disease prevention, as well as develop guidelines for large groups of patients with the same disease.” This definition originated from a 2006 issue of the Journal of the American Medical Association (JAMA).1 The evidence is retrieved from the published medical literature, and it is ranked according to the study design or type of paper.
Pharmacists should be well versed in EBM, so they may answer clinical questions with accuracy. EBM also allows the pharmacist to better scrutinize physician orders so as to identify a more suitable medication or a less expensive alternative.
Levels of Evidence
The study designs are listed in order of importance and strength of the evidence: 1) systematic reviews, 2) randomized controlled trials, 3) cohort studies, 4) case-control studies, 5) case series and case reports, and 6) editorials and expert opinions.2 Some experts place meta-analyses at the very top and animal studies at the very bottom.3 It is helpful to understand the strength of each level of evidence by defining each study design.
Meta-analysis: A meta-analysis is the result of an exhaustive search of the medical literature, selecting only valid, randomized controlled trials pertaining to a specific topic. Meta-analyses are designed with inclusion and exclusion criteria and use complex statistical methodology to combine the results of the studies, as though they came from one large study.3
Systematic Reviews: Systematic reviews are similar to meta-analyses. They result from an exhaustive literature search with the intent of answering some clinical question. The articles are all reviewed, and the results are summarized. The Cochrane Collaboration consists of systematic reviews.2,3
Randomized Controlled Clinical Trials: Randomized controlled clinical trials are experimental, prospective studies. Subjects are randomly allocated to a control group (no intervention) or to an experimental group (those receiving treatment or having a diagnostic test performed). A method to prevent bias is called blinding. This means that the patients do not know whether they are receiving the active treatment or a placebo. Double-blinding is when neither the researcher nor the study participants know who is receiving the experimental treatment or the placebo.2,3
Cohort Studies: Cohort studies differ from randomized controlled clinical studies in that they are observational. Large populations are observed over time to determine if exposure to something (i.e., smoking) or a health condition (i.e., obesity) will result in a certain effect. Cohort studies are not randomized, and there may be other confounding conditions affecting the results. For these reasons cohort studies are less reliable than randomized controlled clinical trials. There are two types of cohort studies, prospective and retrospective. A prospective study follows the participants into the future. For instance, if the researchers wanted to determine if obesity causes heart disease, they would follow a population of obese patients, sometimes for years, to determine what percentage of them got heart disease compared to the “normal” population. This type of study is expensive, and some patients may be lost to follow-up because they drop out.2,3
A retrospective study collects data from the past from hospital charts or patients’ recollections. Although these studies may be cheaper, incomplete charts or erroneous recollections can skew the results.2,3
Case-Control Studies: Unlike cohort studies, case-control studies look at study participants who already have a condition to determine if exposure to something caused the condition. Case-control studies are observational, retrospective studies, so they are vulnerable to the same limitations as retrospective cohort studies. It is also difficult to determine how much exposure may have caused the condition. For instance, when trying to determine if asbestos causes lung cancer, it is likely that patients will not know their exact level of asbestos exposure.2,3
Case Series: A case series is simply a report describing many case reports on the same subject. Although case series are considered very weak evidence, they serve the purpose of introducing the practitioner to rare, unusual conditions.2
Editorials and Expert Opinions: Although editorials and expert opinions play a role in evidence-based practice, keep in mind that they are not studies, and therefore are not considered strong evidence. There is no control group, and therefore the results have no statistical value. However, the experience of experts is valuable and may be necessary to make a clinical decision.2,3
PICO
When looking for an answer to a clinical question, it is important to set it up so that an effective search can be performed. That is where PICO, a model used to formulate clinical questions, comes in (TABLE 1). It consists of four key elements: P = Patient or population (What characteristics does the patient have? What is the disorder or condition of interest?); I = Intervention (What intervention, diagnostic tool, prognostic factor, or exposure is being considered?); C = Comparison (the alternative that is being considered); and O = Outcome (What is the desired outcome?).2,3
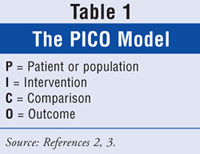
For instance, a physician calls down to the pharmacy and asks the pharmacist which medication has been shown to work better for hypertension in a patient with congestive heart failure, an ACE inhibitor or an angiotensin receptor blocker (ARB)? After determining that the patient has no contraindications to either class of medication, the pharmacist could use the PICO model like this: P = patient with congestive heart failure; I = ACE inhibitor; C = ARB; O = lower blood pressure.
Another example would be a physician who calls down to the pharmacy and asks the pharmacist, “In a patient with a UTI [urinary tract infection] positive for Pseudomonas aeruginosa, would a quinolone be more effective than an aminoglycoside?” P = patient with UTI caused by P aeruginosa; I = aminoglycoside; C = quinolone; O = effective against P aeruginosa. Suppose a nurse asks the pharmacist, “I have a patient who has been on AcipHex for GERD [gastroesophageal reflux disease], but it is so expensive. Can you suggest something that would be cheaper but still relieve GERD?” The pharmacist thinks about it and decides that an alternative could be an H2 blocker. Before recommending it, though, he wants to check the evidence to confirm his suspicion. He consults his resources and develops a question to research: P = patient with heartburn; I = AcipHex (or proton pump inhibitor); C = H2 blocker; O = relief of heartburn.
Other Clinical Concerns
Although the pharmacist will most likely be dealing with questions about therapy, there may be occasions when patients, nurses, or physicians will ask questions from other categories. It is good to be aware of the other medical issues, including diagnosis, etiology or harm, prognosis, prevention, and clinical examination. Certain study designs should be looked at depending on the issue (TABLE 2).
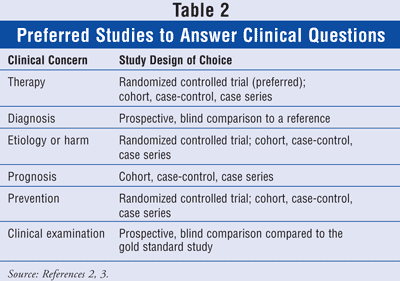
If the question deals with a therapy, then a randomized controlled study is the design of choice. The pharmacist should look for current articles comparing the two therapies. If none can be found, the following choices, in order of value, are cohort, case-control, and case series. If the question deals with a diagnosis, then the study design that should be used is the prospective, blind comparison to a reference. With a question trying to determine etiology or harm, the study designs are (from most valuable to least valuable) randomized controlled trial, cohort study, case-control, and case series. In dealing with an issue of prognosis, the desired studies are cohort, case-control, and case series. The randomized controlled trial is the strongest evidence when trying to answer a question about prevention. If none can be found, then the evidence (strongest to weakest) is the cohort, case-control, and case series. When trying to determine the best way to do a clinical examination, the prospective, blind comparison to the gold standard study is what should be retrieved.2,3
An example might be, “Does the measles-mumps-rubella (MMR) vaccine cause autism in children?” This question falls under the harm category. A randomized controlled trial is not ethical, so it would be unlikely that any would be found. A cohort study would be appropriate, either prospective or retrospective. PICO would be set up like this: P = children over 1 year of age; I = MMR vaccine; C = no MMR vaccine; O = autism.
A physician thought he heard that after a myocardial infarction (MI), aspirin may not improve mortality. He asks the clinical pharmacist, while on rounds, “Does daily aspirin really improve mortality, or is there evidence to the contrary?” This question is looking at a prognosis. When researching the answer, the pharmacist should look for cohort studies first. The question can be set up like this: P = postmyocardial infarction patient; I = an aspirin a day; C = no aspirin; O = improve mortality.
Another question dealing with prognosis might present itself like this: A patient walks up and tells the pharmacist she heard that taking a vitamin E supplement will help prevent her from getting heart disease. Is this true? The pharmacist will first look for cohort studies: P = woman; I = no vitamin E supplement; C = vitamin E; O = Prevent heart disease.
Evaluating the Evidence
It is important to make sure that the articles used are from well-designed studies. There are a few things to look for when evaluating an article. The first is to check whether it is the proper study type for the question you are researching. Next, the actual methods of the study should be examined. There should be a clear research question and a large study population. (A study of 16 patients is not nearly statistically valid as a study with 3,000 patients.) There should be clear inclusion and exclusion criteria listed. There should be a control group,2 and the study should have a clearly defined end point. The researchers should also explain how the study was performed. Even a review article should list the databases searched and exactly which articles were used (inclusion and exclusion criteria).
Once it is determined that the design is sound, the next question is, “Does this apply to my patient?” If the clinician has a patient with a dog bite on the face, but he is finding articles about dog bites on the legs, the articles do not necessarily pertain to his patient.
Most importantly, the study should be free of bias. Bias can happen in many different ways. A randomized control trial should have a participant from the control group who matches (as closely as possible) a participant from the study group.2 For instance, if you have a study participant who is a 58-year-old male weighing 350 lb, the control participant should not be a 23-year-old female weighing 120 lb.
Bias can also occur if the study is not double-blinded. If researchers know which patients are taking the real treatment, their observations may become clouded. Likewise, if the subjects know whether they are taking the active treatment or a placebo, their response to the treatment during the study may not be valid. All randomized controlled studies should be double-blinded, meaning that neither the researcher nor the participant knows who is taking the placebo and who is getting the active therapy.2
Another form of bias is when the end point of the study is based on opinion. An example could be a clinical study comparing a new pain reliever to the gold standard. The subject is asked to determine his pain before the trial starts and then again at the conclusion of the trial. The patient’s level of pain is completely subjective. A similar example might involve a trial determining the efficacy of a new face cream designed to diminish wrinkles. This time the researcher may be biased, since there is no clear end point.2
When evaluating the medical literature, make sure to pay attention to how many subjects were lost during the trial due to dropping out. Eighty percent or more of the subjects should be still participating at the conclusion of the trial.3 If there are less than 80%, bias can occur and render the results invalid. This is especially true for a study with a small sample size to begin with.
Finally, the statistical methodology must be appropriate for the study being done. Statistics can be manipulated to favor the researcher. Just because the statistical methods are listed in the paper does not mean they are appropriate. Health care statistics are a total subject by themselves and beyond the scope of this paper. However, being able to look at a methodology and decide if it is appropriate is well within the pharmacist’s capabilities. It would not be unwise to invest in a book on statistics for the health professional.
Databases
The busy practitioner does not usually have much time to do literature searches for the best evidence on a clinical question. Perhaps consulting a good database may make incorporating evidence-based medicine into his or her practice a little more manageable. There are some very good databases that offer only evidence-based medicine content (TABLE 3). PubMed, maintained by the NLM, has a feature to limit results to only systematic reviews or meta-analysis papers. PubMed is free of charge and available on the Internet.4

The National Guideline Clearinghouse is another free database provided by the U.S. government. It is maintained by the Agency for Healthcare Research and Quality (AHRQ). This database can be searched by disease state and treatment or intervention. There is also an index that alphabetically lists many disease states and interventions.5 This database is a gold mine of information.
Another free database available via the Web is the TRIP database. It is devoted to evidence-based medicine only. The TRIP database has the capacity to be searched by medical images, evidence-based medicine, and patient leaflets. Although at one time there was a subscription fee, this is currently an open-access site, meaning it is free to the public.6 In addition, there are some other very good databases available on a subscription basis (see TABLE 4).

Obstacles to Implementation
There will always be obstacles to implementing evidence-based medicine in your practice. Time is most likely the first thing that comes to mind. Many of the questions the pharmacist gets may not need to be answered immediately. If a physician or nurse calls you with a question, it may be an option to tell them you will research it and get back to them. In a hospital practice, many of these research tools may be readily available and quickly accessed.
Another obstacle may be cost or access to these tools. TABLE 3 lists some free and reliable resources. Another obstacle may be the pharmacist’s lack of interest in pursuing this avenue of pharmaceutical practice. Pharmacists have not traditionally used databases other than the ones their employers adopted for their dispensing duties. Change is always difficult, but these databases are not hard to learn. If time is an issue, which it usually is, the databases listed in TABLE 4 do all the work for you. There are easy tutorials, but the pharmacist must be motivated to use these tools.
With pharmacists moving toward a role of medication therapy management rather than just dispensing medication, it is crucial for the practitioner to keep up with the latest evidence and guidelines.
REFERENCES
1. The National Library of Medicine’s Medical Subject Headings (MeSH). www.ncbi.nlm.nih.gov/sites/
2. Evidence-based practice in the health sciences: evidence-based practice in pharmacy tutorial. Information Services Department of the Library of the Health Sciences–Chicago, University of Illinois at Chicago. http://ebp.lib.uic.edu/
3. Evidence-based medicine tutorial. Health Sciences Library at the University of North Carolina–Chapel Hill. www.hsl.unc.edu/Services/
4. PubMed. www.pubmed.com. Accessed May 25, 2009.
5. National Guideline Clearinghouse. www.guideline.gov. Accessed May 25, 2009.
6. TRIP database. www.tripdatabase.com/index.
To comment on this article, contact rdavidson@jobson.com.






