US Pharm. 2014;39(1):HS2-HS.
ABSTRACT: Cerebral vascular disease is the fourth leading cause of death in the United States. Hemorrhagic stroke accounts for 13% of all stroke cases per year. The use of oral anticoagulants leads to a 7- to 10-fold higher risk of spontaneous intracranial hemorrhage. With the introduction of the oral direct thrombin inhibitor dabigatran and the factor Xa inhibitors rivaroxaban and apixaban come questions about emergent reversal in the presence of hemorrhagic stroke. There are no currently available antidotes to any of the newer oral anticoagulants. The use of blood products and other therapies for acute management and reversal is explored.
Cerebral vascular disease is currently the fourth leading cause of death in the United States.1 It has been reported that a cerebral vascular accident (CVA) occurs every 40 seconds in the U.S. at an estimated rate of 795,000 new or recurrent cases each year. Of these cases, 13% are classified as being hemorrhagic in nature, with bleeding occurring in either the intracerebral or subarachnoid space.2
Intracerebral hemorrhage (ICH) is defined as a focal collection of blood within the brain parenchyma or ventricular system that is not caused by trauma.3 It is the most common cause of hemorrhagic stroke in the U.S., accounting for 10% of all stroke cases per year.4 A subarachnoid hemorrhage (SAH) is defined as bleeding into the subarachnoid space, the space between the arachnoid membrane and the pia mater of the brain or spinal cord.3 It is estimated that SAH accounts for 3% of all stroke cases in the U.S.; however, this may be inaccurate due to SAH often being classified as simply intracranial hemorrhage that encompasses any bleeding within the skull.4
There are many risk factors that may increase a person’s likelihood of experiencing a hemorrhagic stroke. Age, ethnicity, gender, and genetics are nonmodifiable factors correlated to increased risk of hemorrhage. It has been well documented that with each decade of life after age 55 years, the risk of intracerebral hemorrhage doubles.5 When comparing ethnicities, African Americans and Hispanics have been shown to have a higher risk of intracerebral hemorrhage in relation to non-Hispanic whites.6 Stroke, including those hemorrhagic in nature, is more common among men than women. The exception to this appears to be during the fourth and fifth decades of life (ages 35-44 years) in which women have been shown to have a greater risk. Genetics, specifically familial history of stroke, increases a person’s risk of CVA by approximately 30%.5
There are a number of modifiable risk factors for intracranial hemorrhage, with hypertension likely being the most important. Other risk factors include alcohol intake (in which risk increases as intake increases), low levels of serum cholesterol (an inverse association has been noted), drug abuse (specifically sympathomimetics such as cocaine), cigarette smoking (which increases SAH risk 2- to 4-fold), and anticoagulation.7
Oral Anticoagulants
Persons who use oral anticoagulants (OACs) have a 7- to 10-fold higher risk of spontaneous ICH compared with those not receiving treatment.4 It is estimated that nearly 12% of all ICHs may be related to the use of OACs, with mortality occurring in 50% of patients with anticoagulation-related ICH.4,8,9 The vitamin K antagonist warfarin has been the most widely used oral anticoagulant since its introduction in 1954. Warfarin is indicated for both treatment and prophylaxis of thromboembolic disorders, including the prevention of cardioembolic stroke secondary to atrial fibrillation. The risk of hemorrhagic stroke with warfarin is directly related to the intensity of anticoagulation; however, most warfarin-associated ICHs occur while international normalized ratios (INRs) are within the generally accepted therapeutic range of 2.0 to 3.5.8
Warfarin reversal in acute hemorrhagic stroke is docu-mented in numerous case series and centers around the administration of vitamin K and fresh frozen plasma (FFP). Neither of these methods is ideal for emergency reversal, as they both take several hours to normalize INR. They are, however, useful in restoring the body’s ability to produce clotting factors and reverse the coagulation defect, respectively, providing a sustained effect on the coagulation cascade.10
Reversal Agents
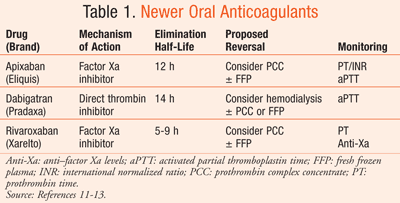
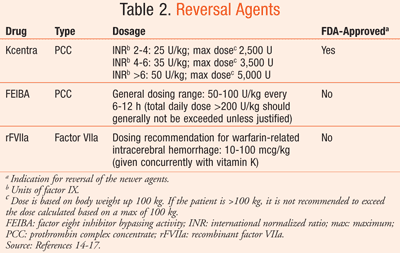
With the introduction of newer OACs come many questions about emergent reversal. The oral direct thrombin inhibitor dabigatran and the factor Xa inhibitors rivaroxaban and apixaban have yet to be on the market for 5 years (TABLE 1).11-13 Though data are far from robust, we present currently proposed options for acute management of newer OACs in acute hemorrhagic stroke, including the reversal agents prothrombin complex concentrates (PCCs), FFP, and recombinant factor VIIa (rFVIIa) (TABLE 2).14-17
Prothrombin Complex Concentrates: PCCs are derived from concentrated pooled plasma products that contain either 3 or 4 vitamin K–dependent clotting factors. Three-factor PCCs contain factors II, IX, and X, while 4-factor PCCs also contain factor VII. In addition to clotting factors, both formulations may include small quantities of protein C and S, heparin, and antithrombin to aid in the reduction of thrombo-genicity. Currently, the only 3-factor PCCs available in the U.S. are Profilnine SD and Bebulin VH. Unfortunately, evidence for their use as reversal agents has not been established.18,19
Kcentra (prothrombin complex concentrate [human]) and FEIBA (factor eight inhibitor bypassing activity) are the two 4-factor PCCs currently available in the U.S. (TABLE 2).14,15 FEIBA is the only commercially available activated 4-factor PCC, as it contains factor VII primarily in the activated form.20 Kcentra was approved by the FDA in April 2013.14 The primary concern with using activated PCC is the subsequent increased thrombotic risk.20 The safety and efficacy of 4-factor PCCs in hemorrhagic patients has not been assessed in large systematic studies. The small body of evidence that does exist is limited to animal models, healthy volunteers, and case reports.
Several animal models have been developed to investigate 4-factor PCCs as viable reversal agents, but it is difficult to extrapolate animal data for human use. Both activated and nonactivated PCCs were tested in a rat-tail bleeding model in which supratherapeutic doses of dabigatran were administered. Bleeding time was corrected to baseline within 5 minutes of PCC administration. Conversely, thrombin time (TT), activated partial thromboplastin time (aPTT), and ecarin clotting time (ECT) did not correct after treatment.21
The efficacy of nonactivated PCC was assessed in a model where mice received dabigatran at a dose that provided systemic anti-coagulation. Following successful anticoagulation, induction of intracranial hemorrhage was performed. PCC was administered at 100 U/kg 30 minutes after the initiation of the hemorrhage, which led to reduced hematoma expansion and tail-vein bleeding time.22
To date, only one small study involving nonactivated PCC has been performed in humans. A randomized, placebo-controlled, crossover study involving 12 healthy men not actively bleeding evaluated the use of nonactivated PCC as a reversal agent in patients taking either rivaroxaban 20 mg twice daily or dabigatran 150 mg twice daily.23 After 2 days of anticoagulation therapy, patients were treated with 4-factor PCC at 50 U/kg or a saline placebo. The prothrombin time was immediately reversed in the rivaroxaban-treated patients. In the dabigatran group, PCC had no effect on aPTT, TT, or ECT. The authors concluded that nonactivated PCC at 50 U/kg immediately and completely reverses the anticoagulant effect of rivaroxaban in healthy individuals but has no effect on the anticoagulant effects of dabigatran.23
There are several published case reports involving the use of PCC in patients with active bleeds. In most cases a multi-intervention/drug approach was taken, yielding conflicting results and making it difficult to determine if PCC alone is a viable option. In one such case report, FFP, PCC, and rFVIIa failed to correct life-threatening bleeding secondary to dabigatran in elderly patients.24 In another fatal case report, a dabigatran-treated patient presented with a traumatic subdural hematoma. The patient was unsuccessfully treated with PCC and other procoagulant factors.25 Conversely, one case reported that postsurgical bleeding was managed successfully with 4-factor PCC and other procoagulants in a patient receiving dabigatran.26
An ex-vivo crossover study was done in blood samples of 10 healthy white men who took a single dose of rivaroxaban 20 mg and dabigatran 150 mg. The samples were treated with nonactivated PCC, rFVIIa, or FEIBA. The results concluded that only FEIBA reversed all of the thrombin generation tests in the rivaroxaban samples and that both the nonactivated PCC and FEIBA increased thrombin generation in the dabigatran samples.27
rFVIIa and FFP: rFVIIa is a prohemostatic agent that can be used for patients with complicated coagulation disorders.16,17 FFP is a blood product that contains all coagulation factors.28 It is kept frozen during storage and therefore must be thawed for 15 to 20 minutes before it can be used. ABO blood compatibility testing is also required before admin-istration. Due to these obstacles, immediate administration in an emergent situation may not be possible.29
A case of life-threatening gastrointestinal (GI) and incisional bleeding secondary to accumulation of dabigatran was reported by Harinstein et al.30 FFP, rFVIIa, and cryoprecipitate were administered without benefit. Furthermore, a 92-year-old man developed profuse rectal bleeding secondary to an unidentified gastric ulcer after taking one dose of dabigatran. He died 7 days later despite treatment with packed red blood cells (PRBCs), FFP, platelets, and vitamin K.31 In two other cases (a lower GI bleed and a pericardial effusion), bleeding was controlled after administration of PRBCs, platelets, and FFP.32 The use of FFP and rFVIIa as reversal agents for factor Xa inhibitors has not been evaluated in humans.29
Hemodialysis
Hemodialysis or hemoperfusion is considered an option for removal of dabigatran. Thirty-five percent of dabigatran is protein-bound; therefore, the remaining unbound portion can be removed via dialysis. One open-label study found that dialysis removes 60% of serum dabigatran in 2 or 3 hours.33 Although an option, it may not be possible to place an unstable patient on dialysis. Rivaroxaban and apixaban are highly protein-bound, 92% to 95% and 87%, respectively.12,13 Therefore, dialysis is not an option with their use.
Investigational Therapies
There are new drug entities currently under investigation to reverse the effects of the new OACs. One such entity is a modified, inactive form of factor Xa that retains an affinity to factor Xa inhibitors.34 Monoclonal antibodies to direct thrombin inhibitors are also being investigated.35 Though the approval of these agents for human use may be years away, if found effective they may ease concerns many practitioners have with the use of newer OACs.
Conclusion
Appropriate management of acute hemorrhagic stroke in the presence of onboard OACs remains a mystery. Unlike with warfarin, there are no definitive reversal agents for direct thrombin inhibitors or factor Xa inhibitors. Despite the lack of definitive data, a few things are certain. First, discontinuation of the OAC is paramount. The use of hemodialysis in patients receiving dabigatran is also an option. Four-factor PPCs, such as Kcentra, seem to be the most promising emergent treatment. Though efficacy in the reversal of dabigatran is still uncertain, its use in patients receiving factor Xa inhibitors seems promising. The use of FFP and rFVIIa alone or in combination appears to be ineffective in the reversal of dabigatran. Their use will likely be either institution or practitioner-specific.
REFERENCES
1. Minino AM. Death in the United States, 2011. NCHS Data Brief. 2013;115:1-8.
2. Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease
and stroke statistics–2010 update: a report from the American Heart
Association. Circulation. 2010;121:e46-e215.
3. Sacco RL, Kasner SE, Broderick JP, et al. An updated
definition of stroke for the 21st century: a statement for healthcare
professionals from the American Heart Association/American Stroke
Association. Stroke. 2013;44:2064-2089.
4. Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of
ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and
risk factors. Neurologic Clinics. 2008;26:871-895.
5. Goldstein P, Elalamy I, Huber K, et al. Rivaroxaban and
other non-vitamin K antagonist oral anticoagulants in the emergency
treatment of thromboembolism. Int J Emerg Med. 2013;6:25.
6. Labovitz DL, Halim A, Boden-Albala B, et al. The
incidence of deep and lobar intracerebral hemorrhage in whites, blacks,
and Hispanics. Neurology. 2005;65:518-522.
7. Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors
for intracerebral hemorrhage in the general population: a systematic
review. Stroke. 2003;34:2060-2065.
8. Aguilar MI, Kuo RS, Freeman WD. New anticoagulants
(dabigatran, apixaban, rivaroxaban) for stroke prevention in atrial
fibrillation. Neurologic Clinics. 2013;31:659-675.
9. Steiner T, Rosand J, Diringer M. Intracerebral
hemorrhage associated with oral anticoagulant therapy: current practices
and unresolved questions. Stroke. 2006;37:256-262.
10. Caceres JA, Goldstein JN. Intracranial hemorrhage. Emergy Med Clin North Am. 2012;30:771-794.
11. Eliquis (apixaban) package insert. Princeton, NJ: Bristol-Myers Squibb Co; December 2012.
12. Pradaxa (dabigatran) package insert. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; December 2013.
13. Xarelto (rivaroxaban) package insert. Titusville, NJ: Janssen Pharmaceuticals, Inc; August 2013.
14. Kcentra (prothrombin complex concentrate [human]) package insert. Kankakee, IL: CSL Behring GmbH; November 2013.
15. FEIBA (factor eight inhibitor bypassing activity)
package insert. Westlake Village, CA: Baxter Healthcare Corp; February
2011.
16. Ilyas C, Beyer GM, Dutton RP, et al. Recombinant factor VIIa for warfarin-associated intracranial bleeding. J Clin Anesth. 2008;20:276-279.
17. Freeman WD, Brott TG, Barrett KM, et al. Recombinant
factor VIIa for rapid reversal of warfarin anticoagulation in acute
intracranial hemorrhage. Mayo Clin Proc. 2004;79:1495-1500.
18. Miyares MA, Davis K. Newer oral anticoagulants: a
review of laboratory monitoring options and reversal agents in the
hemorrhagic patient. AJHP. 2012;69:1473-1484.
19. Patanwala AE, Acquisto NM, Erstad BL. Prothrombin complex concentrate for critical bleeding. Ann Pharmacother. 2011;45:990-999.
20. Gass JA, Weeks PA. Managing new oral anticoagulants in the intensive care unit. Crit Care Nurs Q. 2013;36:390-399.
21. van Ryn J, Stangier J, Haertter S, et al. Dabigatran
etexilate—a novel, reversible, oral direct thrombin inhibitor:
interpretation of coagulation assays and reversal of anticoagulant
activity. Thrombosis Haemostasis. 2010;103:1116-1127.
22. Zhou W, Schwarting S, Illanes S, et al. Hemostatic
therapy in experimental intracerebral hemorrhage associated with the
direct thrombin inhibitor dabigatran. Stroke. 2011;42:3594-3599.
23. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al.
Reversal of rivaroxaban and dabigatran by prothrombin complex
concentrate: a randomized, placebo-controlled, crossover study in
healthy subjects. Circulation. 2011;124:1573-1579.
24. Lillo-Le Louet A, Wolf M, Soufir L, et al.
Life-threatening bleeding in four patients with an unusual excessive
response to dabigatran: implications for emergency surgery and
resuscitation. Thrombosis Haemostasis. 2012;108:583-585.
25. Harvey P. Risky anticoagulants: meds have the potential to turn minor trauma into a major disaster. JEMS. 2012;37:30-31.
26. Mastrobuoni S, Robblee JA, Boodhwani M. Spontaneous ascending aortic intramural haematoma in a patient on dabigatran. Interact Cardiovasc Thorac Surg. 2012;15:299-300.
27. Marlu R, Hodaj E, Paris A, et al. Effect of
non-specific reversal agents on anticoagulant activity of dabigatran and
rivaroxaban: a randomised crossover ex vivo study in healthy
volunteers. Thromb Haemost. 2012;108:217-224.
28. Benjamin RJ, McLaughlin LS. Plasma components: properties, differences, and uses. Transfusion. 2012;52(suppl 1):9s-19s.
29. Nitzki-George D, Wozniak I, Caprini JA. Current state
of knowledge on oral anticoagulant reversal using procoagulant factors. Ann Pharmacother. 2013;47:841-855.
30. Harinstein LM, Morgan JW, Russo N. Treatment of dabigatran-associated bleeding: case report and review of the literature. J Pharm Pract. 2013;26:264-269.
31. Kernan L, Ito S, Shirazi F, Boesen K. Fatal gastrointestinal hemorrhage after a single dose of dabigatran. Clin Toxicol (Phila). 2012;50:571-573.
32. Dy EA, Shiltz DL. Hemopericardium and cardiac tamponade associated with dabigatran use. Ann Pharmacother. 2012;46:e18.
33. Stangier J, Rathgen K, Stahle H, Mazur D. Influence of
renal impairment on the pharmacokinetics and pharmacodynamics of oral
dabigatran etexilate: an open-label, parallel-group, single-centre
study. Clin Pharmacokinet. 2010;49:259-268.
34. Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific
antidote for reversal of anticoagulation by direct and indirect
inhibitors of coagulation factor Xa. Nat Med. 2013;19:446-451.
35. Majeed A, Schulman S. Bleeding and antidotes in new oral anticoagulants. Best Pract Res Clin Haematol. 2013;26:191-202.
To comment on this article, contact rdavidson@uspharmacist.com.





