US Pharm. 2014;39(5):47-51.
ABSTRACT: Long QT syndrome (LQTS), in which cardiac repolarization is delayed following a heartbeat, can be measured by ECG. Universal ECG screening is not standard practice in the United States, and many children with congenital LQTS go undiagnosed. LQTS creates an electrophysiological environment that favors the development of serious ventricular dysrhythmias. Pharmacist awareness is important because drugs can play a major role in exacerbating underlying LQTS or causing acquired LQTS. Common symptoms of LQTS include fainting spells, heart palpitations, and seizures. Most importantly, LQTS is a major risk factor for ventricular arrhythmias, which can quickly deteriorate, leading to cardiac arrest and sudden cardiac death.
Long QT syndrome (LQTS), a heart condition in which cardiac repolarization is delayed following a heartbeat, can be detected by measuring the QT interval on an ECG. A long QT interval creates an electrophysiological environment that favors the development of life-threatening, prolonged ventricular dysrhythmias. Because drugs can figure significantly in the development of symptoms in patients with LQTS, pharmacist awareness is important. Common manifestations are heart palpitations, fainting spells, and seizures. Sudden cardiac death (SCD), to which LQTS is a major contributor, is estimated to occur in as many as 6.2 of 100,000 children per year.1 SCD also accounts for 50% of all adult deaths from cardiovascular (CV) disease, with 80% to 85% of cases caused by acute ventricular arrhythmias.2 Given many recent FDA warnings about drugs that can prolong the QT interval and potentially increase the risk of ventricular dysrhythmias, it is becoming increasingly more important for pharmacists to heighten their awareness of this condition in order to help patients use their medications safely and effectively.3,4
The QT Interval and LQTS
The QT segment of the larger PQRST(U) complex of the ECG represents the duration of ventricular depolarization and subsequent repolarization (i.e., the heartbeat). A heart with a rate of 60 bpm beats an average of once per second; or, as depicted on an ECG, the rhythm repeats every 1,000 msec. LQTS is typically diagnosed by the measurement (and mathematical correction) of an extended QT interval on an ECG. FIGURE 1 demonstrates, in simplified fashion, how a prolonged QT interval may appear on a single lead of an ECG. Measurement can be affected by many factors, including which lead is used and what is determined to be the exact end of the T wave. On a 12-lead ECG, the lead showing the longest QT interval is the most sensitive for making the calculation. A newer, more sophisticated, and perhaps more sensitive and specific approach for the detection of acquired and congenital long QT syndrome is to apply the root mean square ECG.5 This newer method has yet to become the standard of practice.
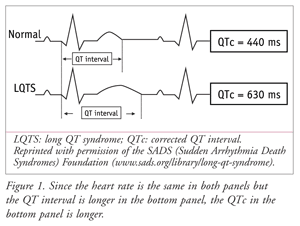
Most clinicians consider a corrected QT interval (QTc) of >440 msec for males and >450 msec for females to be prolonged. As the heart rate increases, the QT interval shortens. Therefore, it is important to calculate QTc in patients with elevated heart rates so that the QT interval is not underestimated. In the absence of provoking factors (e.g., QT-prolonging drugs), a QTc >470 msec in males and >480 msec in females is suggestive of LQTS. Patients with a QT interval longer than normal, whether it is borderline (440-470 msec in males and 450-480 msec in females) or suggestive of LQTS, should undergo repeat or additional diagnostic testing.6
The Impact of LQTS
LQTS, hypertrophic cardiomyopathy, and Wolff-Parkinson-White syndrome are the most common causes of SCD in children that can be detected by ECG. Universal ECG screening is not standard practice in the United States, so many children with congenital LQTS are not diagnosed.7 LQTS may be inherited or acquired. Acquired LQTS, which is usually drug-induced, is more common than inherited LQTS.2
The prevalence of inherited LQTS in the U.S. is estimated to be one in 2,000 to 2,500 live births.2
It is unknown precisely how many deaths are caused by the acquired form
or what is the exact risk of developing LQTS after taking a drug, since
these factors are unpredictable and have not been thoroughly
researched; however, a decade ago, the single most common reason for FDA
withdrawal of a prescription drug from the market was the associated
risk of QT-interval prolongation.8 Many individuals with
inherited LQTS are asymptomatic, and the condition is often discovered
incidentally upon ECG or after an episode has occurred. Subtypes of
inherited LQTS are caused by mutations in the genes that encode for
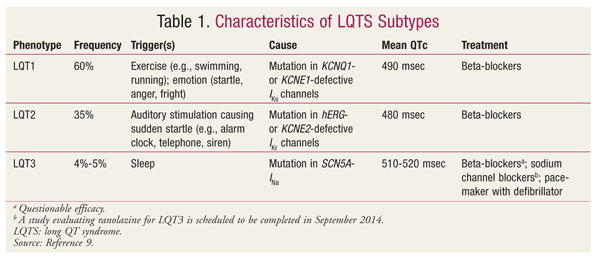
cardiac ion channels (TABLE 1).9 Individuals with
inherited LQTS may remain asymptomatic for a lifetime, or they may
experience symptoms from as early as the first months of life to as late
as middle age.2
LQTS can be detected in children through ECG, although widespread routine screening in asymptomatic patients is not currently recommended in the U.S.7 The prognosis for untreated patients presenting with syncope is a 20% chance of death after 1 year, and a 50% chance within 10 years. In symptomatic patients, episodes may be brought on by QT-prolonging drugs, ill-advised drug doses or combinations, physical activity such as swimming, or being startled; an episode may even occur while sleeping.2
Screening for LQTS
As of early 2014, only one country worldwide was found to require universal ECG screenings. For more than 25 years, Italy has mandated medical screening for any individual wishing to participate in competitive sports. This screening includes personal and family history, physical examination, and resting and exercise 12-lead ECG for detecting cardiovascular (CV) abnormalities that would put a person at risk for ventricular arrhythmias.10
Universal screening for phenylketonuria, which has an estimated prevalence of one per 10,000 to one per 12,000 live births, is recommended for newborns in the U.S.11 When phenylketonuria prevalence is compared with that of congenital LQTS (one in 2,000-2,500 live births), it would appear reasonable to adopt universal screening for LQTS. However, factors other than prevalence—like the sensitivity and specificity of ECG or other screening tests—also must be considered.
A recent meta-analysis estimated that, at the maximum accuracy point, the number needed to screen to detect one case of LQTS by ECG exceeded 16,000.7 However, at this accuracy point, 14% of patients who actually had LQTS were missed (false-negatives), and more than 2,000 false-positives (for each true positive) were detected. Since the specificity was low (high rate of false-positives), if the maximum specificity point were to be used instead of maximum accuracy, false-positives could be reduced to 135 for each true LQTS case detected, but the number needed to screen to detect one case would increase to 135,000, and 91% of cases would be missed.6 Therefore, it is understandable why universal screening has not been implemented in the U.S.
Screening Prior to Initiating Certain Drugs
In 2008, the American Heart Association (AHA) released a statement recommending that screening be performed prior to initiation of stimulant therapy in children with attention-deficit/hyperactivity disorder (ADHD). Shortly thereafter, the American Academy of Pediatrics (AAP) released a joint statement with the AHA recommending that children with ADHD be assessed with a targeted history and cardiac examination, but that an ECG should be performed only if indicated. A recent study, however, found that only 48% of more than 800 pediatricians completed an in-depth cardiac history and physical, and that only 14.7% ordered an ECG prior to initiating stimulant therapy.12 Most recently, the AAP has withdrawn recommendations for pretreatment ECG screening, except for medications that clearly heighten the risk of ventricular arrhythmias.13 Presumably, this exception refers to drugs that are contraindicated in LQTS patients.
Warnings Issued and Actions Taken
Recent warnings have been issued by the FDA to inform practitioners about CV risks associated with certain medications.3,4 Unfortunately, these warnings come with no recommendations on how to assess risk, instead simply advising that patients discuss any questions or concerns with their healthcare provider. This leaves healthcare practitioners, and particularly pharmacists, in a quandary about how to incorporate information on CV risks into clinical practice. There are a number of questions that could logically be asked: Is there a way to screen or risk-stratify to determine who is at higher risk for developing a life-threatening ventricular arrhythmia? Have the risks of significant QT prolongation and the corresponding development of ventricular dysrhythmia been quantified? Has a clinically significant degree of QT prolongation been defined? Should healthcare practitioners routinely require ECG before initiating certain medications?
Now that all new drugs must undergo QT-prolongation studies prior to FDA approval, the FDA has formalized 10-msec QT prolongation as the threshold for regulatory concern.14 It is common for drugs to evoke a dose-response relationship according to the degree of QT-interval prolongation. For example, it is well established that class III antiarrhythmics cause QT prolongation by extending the duration of cardiac-action potential. The best example is sotalol, which is classified as both a class II and a class III antiarrhythmic. Sotalol, which has been studied in children for treatment of supraventricular and ventricular arrhythmias at three different body-surface area–based doses (10, 30, and 70 mg/m2), demonstrated both dose-related and body type–related differences in QT prolongation.15 This knowledge, as well as new information on several drugs, has led the FDA to restrict certain drugs to new maximum doses, apparently targeting their established 10-msec threshold (TABLE 2).
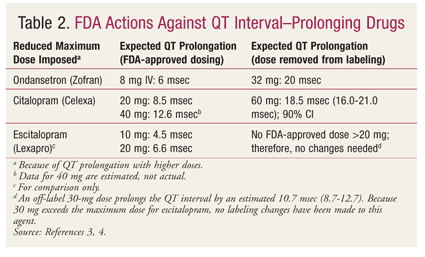
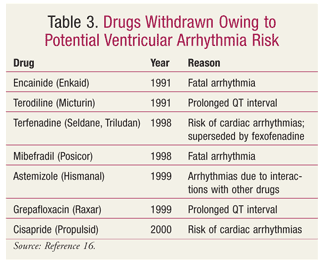
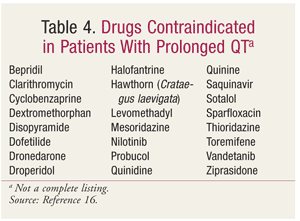
FDA actions against drugs that prolong the QT interval have included reducing the maximum dose (TABLE 2), withdrawing the drug from the market (TABLE 3), and labeling the drug as contraindicated in patients with LQTS (TABLE 4).14,16,17 The maximum dose of citalopram has been restricted to 20 mg in patients who slowly metabolize citalopram by the isoenzyme CYP2C19, whether due to genetics (i.e., poor CYP2C19 metabolization) or to a drug interaction (i.e., concurrent use with a CYP2C19 inhibitor, such as omeprazole).18
The FDA issued a warning in response to a 2012 study that reported increased CV mortality with azithromycin versus amoxicillin use.19,20 The azithromycin label has been updated with a brief statement on azithromycin’s propensity to prolong the QT interval at doses of 500, 1,000, and 1,500 mg. The FDA noted that azithromycin can cause abnormal changes in the heart’s electrical activity that may result in a potentially fatal irregular heart rhythm, and that patients with known risk factors (e.g., existing QT-interval prolongation, low potassium or magnesium blood levels, slower-than-normal heart rate, or the use of certain drugs that treat abnormal heart rhythms) are at greater risk for developing this condition.20
Assessing Drug-Induced Ventricular Dysrhythmia Risk
On a per capita basis, U.S. residents appear to be the world’s foremost consumers of healthcare services. Per capita healthcare costs in the U.S. exceed the median per capita costs of 11 comparator nations by 250%, and those of the nearest comparator by 150%.21 Given recent warnings about citalopram doses and drug interactions, as well as long-standing precautions about arrhythmias from trazodone and tricyclic antidepressants, it is noteworthy than between 2005 and 2008, 11% of Americans aged 12 years and older were taking an antidepressant.4,21 It is widely acknowledged that all medications are associated with risks—many that are known, but also a great number that are unknown or debated. Since only drugs approved by the FDA in the last decade or so have been required to undergo QT-prolongation testing as a part of the New Drug Application, it is not necessarily known whether some older drugs may prolong the QT interval.
Over the last few years, the FDA has issued CV warnings for a variety of drugs in the form of black box warnings, letters to healthcare professionals, alterations in prescribing information, and even restricted access to certain drugs. This has required changes in practice, sometimes before the availability of sufficient or convincing data. The FDA’s premature action against rosiglitazone (proposed association with myocardial infarction), followed by the recent removal of this restriction once better evidence became available, serves as a potent recent reminder of this agency’s reactive tendency.
Treatment and Management
LQTS treatment may include medications, medical devices or surgery, or simple lifestyle changes. In most cases, patients are advised to avoid the specific triggers that usually precede an event, but if this is inadequate for symptom control, medication may be necessary. Since sleep and startle are precipitating factors for ventricular arrhythmias in patients with LQTS (depending on LQTS type), some triggers cannot be easily avoided. Beta-adrenergic receptor antagonists (i.e., beta-blockers) are the most common class of medications used to manage LQTS symptoms. If needed, a defibrillator, which can deliver an electrical shock in order to restore heart rhythm, may be surgically implanted in the chest. For patients who continue to have symptoms while on beta-blockers or who otherwise appear to be at risk for life-threatening arrhythmias, left cardiac sympathetic denervation is another option.22
Pharmacist’s Role
Pharmacists are placed in a challenging situation when assessing or explaining the risks and associated warnings for drugs that modestly prolong the QT interval. Without a standardized screening program for LQTS in the U.S., pharmacists might be expected to presuppose that any given patient could be the estimated one in 2,500 who is predisposed to a prolonged QT interval. Despite the FDA’s warning, azithromycin obviously continues to have an important role in treating certain infections (especially pertussis) in the pediatric population. For a drug (or drug dose) known to significantly prolong the QT interval, and especially for a drug known to be associated with the risk of ventricular arrhythmias, the pharmacist should recommend both baseline and follow-up ECGs, or at least investigate whether an ECG has ever been performed in the patient. If this information is not available, it may be useful to ask the patient about signs of LQTS (e.g., syncope) or family history of sudden cardiac death, although these screening questions probably lack sufficient sensitivity and specificity.
Despite the poorly quantified (or even qualified) risk, pharmacists should be cautious and abide by maximum dose recommendations, avoid bad drug combinations, and help guide therapy toward alternatives to QT-prolonging agents when equally cost-effective therapeutic agents are available. Pharmacists can also monitor for some of the milder symptoms of LQTS, especially in patients taking QT-prolonging drugs, and refer the patient to an appropriate healthcare practitioner if there is any suspicion of ventricular arrhythmias.
REFERENCES
1. Gajewski KK, Saul JP. Sudden cardiac death in children and adolescents (excluding Sudden Infant Death Syndrome). Ann Pediatr Cardiol. 2010;3:107-112.
2. van Noord C, Eijgelsheim M, Stricker BH. Drug- and non-drug-associated QT interval prolongation. Br J Clin Pharmacol. 2010;70:16-23.
3. FDA. FDA Drug Safety Communication: new information regarding QT
prolongation with ondansetron (Zofran).
www.fda.gov/Drugs/DrugSafety/ucm310190.htm. Accessed January 30, 2014.
4. FDA. FDA Drug Safety Communication: revised recommendations for
Celexa (citalopram hydrobromide) related to a potential risk of abnormal
heart rhythms with high doses.
www.fda.gov/Drugs/DrugSafety/ucm297391.htm. Accessed January 30, 2014.
5. Lux RL, Sower CT, Allen N, et al. The application of root mean
square electrocardiography (RMS ECG) for the detection of acquired and
congenital long QT syndrome. PLoS One. 2014;9:e85689.
6. SADS Foundation. Long QT syndrome. www.sads.org/library/long-qt-syndrome. Accessed January 30, 2014.
7. Rodday AM, Triedman JK, Alexander ME, et al. Electrocardiogram
screening for disorders that cause sudden cardiac death in asymptomatic
children: a meta-analysis. Pediatrics. 2012;129:3999-1010.
8. Roden DM. Drug-induced prolongation of the QT interval. New Engl J Med. 2004;350:1013-1022.
9. ClinicalTrials.gov. Efficacy study of sodium channel blocker in
LQT3 patients. http://clinicaltrials.gov/show/NCT01648205. Accessed
January 30, 2014.
10. Sofi F, Capalbo A, Pucci N, et al. Cardiovascular evaluation,
including resting and exercise electrocardiography, before participation
in competitive sports: cross sectional study. BMJ. 2008;337:a346.
11. Koch RK. Issues in newborn screening for phenylketonuria. Am Fam Physician. 1999;60:1462-1466.
12. Leslie LK, Rodday AM, Saunders TS, et al. Cardiac screening prior
to stimulant treatment of ADHD: a survey of US-based pediatricians. Pediatrics. 2012;129:222-230.
13. Perrin J, Friedman R, Knilans TK. Cardiovascular monitoring and
stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122:451-453.
14. FDA. ICH E14 Step 2: The Clinical Evaluation of QT/QTc Interval
Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs.
www.fda.gov/ohrms/dockets/dockets/04d0377/04D-0377-EC7-Attach-1.pdf.
Accessed January 30, 2014.
15. Saul JP, Ross B, Schaffer MS, et al. Pharmacokinetics and
pharmacodynamics of sotalol in a pediatric population with
supraventricular and ventricular arrhythmia. Clin Pharmacol Ther. 2001;69:145-157.
16. CDER 2005 Report to the Nation: Improving Public Health Through Human Drugs. Rockville, MD: Food and Drug Administration; 2005.
17. Clinical Pharmacology [subscription database]. Drugs absolutely
contraindicated for QT prolongation. www.clinicalpharmacology.com.
Accessed January 30, 2014.
18. Celexa (citalopram hydrobromide) product information. St. Louis, MO: Forest Pharmaceuticals, Inc; November 2013.
19. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
20. FDA. FDA Drug Safety Communication: azithromycin (Zithromax or
Zmax) and the risk of potentially fatal heart rhythms.
www.fda.gov/drugs/drugsafety/ucm341822.htm. Accessed April 2, 2014.
21. Pratt LA, Brody DJ, Gu Q. Antidepressant use in persons aged 12 and over: United States, 2005-2008. NCHS Data Brief. 2011;76:1-8.
22. National Heart Lung and Blood Institute. Long QT syndrome.
swww.nhlbi.nih.gov/health//dci/Diseases/qt/qt_treatments.html. Accessed
January 30, 2014.
To comment on this article, contact rdavidson@uspharmacist.com.





