US Pharm. 2015;40(3):HS8-HS12.
ABSTRACT: An operating-room (OR) pharmacist is a great asset to the perioperative team. The establishment of nurse-pharmacist teams in the perioperative area can reduce the incidence of adverse drug events because the pharmacist can review orders prior to administration. OR pharmacists can have a significant effect on hospital compliance with Surgical Care Improvement Project measures. Several regulatory compliance processes can be monitored and addressed daily by OR pharmacists. Initiating new processes and standardizing anesthesia drug trays can decrease medication errors, improve organization of anesthesia medications, and encourage safe injection practices. A key role of the OR pharmacist is to manage narcotic dispensing and reconciliation processes that inhibit drug diversion. Inclusion of a pharmacist on the multidisciplinary OR team should be standard practice in all hospitals.
Given the complexities of current surgery processes, the presence of a pharmacist on the multidisciplinary team is vital to the overall success of the perioperative period. Operating-room (OR) pharmacists assist daily with medication dosing, selection, and dispensing; compounded sterile preparations; Surgical Care Improvement Project (SCIP) guideline compliance; cost-containment practices; narcotic dispensing and diversion methods; pharmaceutical waste disposal; and regulatory compliance. See TABLE 1 for useful online resources.

Benefits of Pharmacist Collaboration
The use of nurse-pharmacist teams has been reported to prevent adverse drug events during the medication-reconciliation process upon hospital admission and discharge.1 The same type of collaboration is beneficial in the perioperative period. As a member of the first-line multidisciplinary team, the pharmacist can relieve the nursing staff of some responsibilities by reviewing preoperative medications prior to administration for appropriate selection (specifically, SCIP procedures); drug allergies; drug-drug interactions; and weight-based, renal, or hepatic dosage adjustments.
The author of this article has noted, at her hospital, the increased frequency of surgeons employing order sets meant to cover all patients undergoing a particular procedure. Although there may be advantages to this method of prescribing, what gets left out of the equation is individualized care for each patient. The most common problem is allergy to medications in the surgeon’s preoperative orders. This is a key opportunity for the pharmacist to intervene and suggest alternative medications, thereby expediting the patient’s surgical-preparation procedures.
The prevalence of documented beta-lactam allergy is notable, constituting a minimum of 10% of all self-reported allergies.2 This author has found this to be one reason that antibiotics are not stocked in automated dispensing cabinets in outpatient or inpatient holding areas in her hospital. This hospital has made it a priority that all preoperative antibiotic orders be reviewed by a pharmacist and entered into the electronic medication-administration record (eMAR) prior to administration. This allows the pharmacist to screen for possible drug-drug interactions or necessary dosage adjustments and review the patient’s documented allergy list.
Frequently, patients reporting an antibiotic allergy are prescribed alternative antibiotics that are less effective, cover a larger scope of antimicrobials, or cost more than the first-line agent.3 When a pharmacist has the opportunity to evaluate a patient’s self-reported allergies, he or she can determine whether the reaction is a true allergy or a possible side effect. This intervention can obviate the need to switch the initial antibiotic of choice to a more costly or less effective alternative.
SCIP Compliance Measures
The OR pharmacist can play a large role in some SCIP compliance measures. SCIP is a nationally recognized effort by several organizations that have collaborated to help minimize complications associated with certain surgical procedures.4 The Centers for Medicare and Medicaid Services and The Joint Commission (TJC) use SCIP guidelines to evaluate hospitals’ achievements with regard to surgery patients.4
The OR pharmacist can make an impact on three main SCIP measures: SCIP-inf-1, SCIP-inf-2, and SCIP-inf-3. These measures are described thusly in TJC’s 2014 Specification Manual for National Hospital Inpatient Quality Measures: SCIP-inf-1, Prophylactic Antibiotic Received Within One Hour Prior to Surgical Incision; SCIP-inf-2, Prophylactic Antibiotic Selection for Surgical Patients; and SCIP-inf-3, Prophylactic Antibiotics Discontinued Within 24 Hours After Surgery End Time.5 The OR pharmacist can influence hospital compliance with these measures.
SCIP-inf-1: This measure involves achieving sufficient antibiotic levels in the serum and tissue to adequately destroy bacteria during the surgical incision.6 Maintaining a healthy degree of communication between pharmacists and nurse anesthetists is key to sustained compliance with SCIP-inf-1. The pharmacy must dispense preoperative antibiotics promptly so that the time of surgical incision is not delayed.
Specifically addressing the perioperative antibiotic ordered during the surgical “time-out” is another excellent way to prevent falling short on SCIP-inf-1. The requirement of a surgical time-out, which was implemented by TJC in 2004, is one of the three elements constituting the Universal Protocol.7 The Universal Protocol was developed to ensure that the correct surgical procedure is being performed on the correct patient and at the correct surgical site.7 A surgical time-out, which occurs in the OR just before the start of the procedure, consists of identifying the patient and recounting the scheduled procedure and surgical-site information, along with any other pertinent information (e.g., positioning, antibiotics, allergies).7
SCIP-inf-2: The antibiotic-selection process outlined in SCIP-inf-2 involves choosing an antibiotic that is safe and cost-effective, has antimicrobial coverage for most intraoperative contaminants specific to the procedure, and minimizes antibiotic resistance.8 The OR pharmacist is equipped with a list of SCIP-recommended antibiotic selections and alternatives for beta-lactam–allergic patients for all SCIP guideline surgery procedures.9 When the surgeon’s preoperative antibiotic orders are relayed to the OR pharmacy, whether via computerized order entry, verbally, or by telephone, fax, or written instruction, the pharmacist evaluates the selection for appropriateness with SCIP guidelines and makes recommendations to alter a noncompliant order. As with any clinical intervention, the pharmacist must document the occurrence and whether the recommendation was accepted by the physician.
SCIP-inf-3: SCIP-inf-3 focuses on preventing antimicrobial-resistant pathogens and decreasing the incidence of Clostridium difficile infections, which have been linked to extended administration of certain antibiotics.10 SCIP-inf-3 recommends discontinuance of antibiotics within 24 hours of the surgery end time, unless certain exclusion criteria are met.10 This advice is based on data that indicate the importance of sustaining therapeutic tissue and serum levels of antibiotics during surgery, but also show that antibiotics provide no added advantage when administered beyond a few hours after wound closure.10
The OR pharmacist can be instrumental in identifying noncompliance with SCIP-inf-3 measures. After being approached to develop a program that would enable the pharmacy to assist in reducing the number of missed SCIP-inf-3 opportunities, this author and her peers created the Pharmacy SCIP Support Project (PSSP). PSSP consists of reviewing the profiles of patients who underwent a designated SCIP procedure the morning after surgery (postoperative day 1); identifying SCIP postoperative antibiotic orders for the nursing staff by placing a comment that is displayed on the medication label and appears in the eMAR; and contacting the physician prior to the 24-hour postoperative deadline in cases where SCIP-inf-3 guidelines have not been satisfied. In conjunction with the implementation of this program, an OR pharmacist was added to the multidisciplinary SCIP team. This pharmacist attends monthly meetings to evaluate recent missed measures and determine areas for improvement. Since initiation of OR pharmacist involvement, missed SCIP-inf-3 measures have been nearly eliminated, with the few remaining occurrences involving physician documentation issues.
Compliance With Medication Management
Regulatory compliance procedures are an ongoing component within hospital systems. The OR pharmacist is conveniently positioned to monitor daily processes throughout the perioperative area to ensure compliance with the hospital’s policies regarding medication management. Some of the more common issues OR pharmacists encounter in the perioperative area are missing beyond-use dates (BUDs) on multidose vials (MDVs); the use of single-dose vials (SDVs) as MDVs; nursing carts left unlocked while unattended; and improperly labeled medication drawn into a syringe for administration.
Part of the OR pharmacist’s job is to educate the perioperative team about the hospital’s medication policies. Many staff nurses are unfamiliar with the specific regulations surrounding medication management. Discussions about SDVs versus MDVs often reveal that most nursing staff were not trained in the difference between them or how to distinguish one from the other. After identifying the problem, the OR pharmacist can collaborate with the nursing leadership team to develop a method that will encourage compliance with BUD labeling. One possible solution is to place a bright, eye-catching auxiliary label on all MDVs that requires date opened, expiration date, and a staff member’s initials. This simple fix allows the OR pharmacist to monitor compliance at a glance while performing daily tasks.
A more difficult challenge for the OR pharmacist is to ensure that medications drawn into a syringe by nurse anesthetists in the OR are labeled according to hospital regulatory standards. One remedy is to design an anesthesia drug tray that can accommodate prefilled syringes of the agents most commonly used during surgery. This introduces the opportunity to adjust the current trays and allows the OR pharmacist to create a more user-friendly tray that will standardize medication placement in every OR.
FIGURE 1A depicts a general-anesthesia drug tray with no standard format or labels indicating what medication is stocked in the drawer. Prior to pharmacy involvement at this author’s hospital, anesthesia drug trays varied slightly in the medication they contained and in drug arrangement, two scenarios that increase the risk of medication errors. A good objective for the OR pharmacist is to create a new anesthesia drug tray that can hold an array of medications that may be necessary throughout surgery. The OR pharmacist should consult with several staff Certified Registered Nurse Anesthetists (CRNAs) and their manager to ensure that the new drug tray effectively meets their needs.
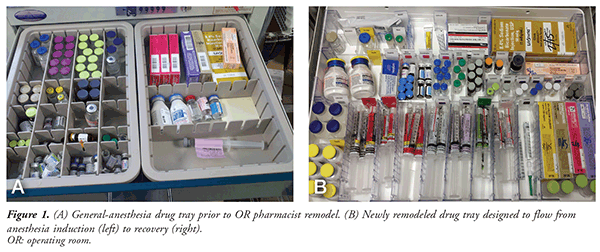
FIGURE 1B is the new anesthesia drug tray developed by the author. The tray is designed to flow from left to right, or from anesthesia induction to recovery. All medication bays are identified with color-specific labels in accordance with the drug-class colors recommended by the American Society of Anesthesiologists (ASA).11 Prefilled syringes are equipped with several visual cues to differentiate the medications: multiple placements of the drug name on the syringe label; the drug name displayed in “Tall Man” lettering, as recommended by the Institute for Safe Medication Practices; and the label color distinctions developed by the American Society for Testing and Materials and endorsed by the ASA.12
In conjunction with designing a new anesthesia drug tray, new processes for stocking, refilling, and outdating the trays must be developed. The responsibility of maintaining the trays lies with the OR pharmacy staff. Some hospitals use a rotation process in which anesthesia technicians deliver fresh trays to the OR and return used trays to the OR pharmacy for replenishment.
Implementation of a new tray process and the use of prefilled syringes are ways to achieve compliance with current safe injection practices and eliminate the use of MDVs in the OR, as suggested by the One & Only Campaign.13 The CDC and the Safe Injection Practices Coalition initiated this public-health campaign to eradicate infections contracted through unsafe injection practices.14 Key objectives of the initiative are to reeducate healthcare providers and standardize the use of “One Needle, One Syringe, Only One Time” for all injections.14 The CDC’s Safe Injection Practices guideline recommends that SDVs be used whenever possible, particularly where medications are administered to multiple patients (e.g., in the OR).15 The guideline advises against stocking MDVs in areas where patients receive immediate treatment.15 Instructing anesthesia technicians to dispose of any open vials in the appropriate waste container while cleaning anesthesia equipment and preparing for the next patient will facilitate compliance with safe injection practice initiatives.
Management of Narcotic-Diversion Processes
Another regulatory compliance measure is management of the hospital’s narcotic-diversion processes. Narcotic diversion is an ongoing concern for all healthcare professions. The accessibility and constant use of narcotics render the OR a prime setting for potential diversion activity. The American Society of Health-System Pharmacists (ASHP) Guidelines on Surgery and Anesthesiology Pharmaceutical Services state that the objective of a controlled-substance system is to inhibit diversion in a functional manner that does not disrupt patient care.16 The OR pharmacist should actively participate in the dispensing of narcotics and the disposal of narcotics waste in the perioperative area. This involvement alone is a deterrent to narcotics diversion.
The ASHP guidelines discuss the two options for dispensing narcotics for surgery: the per-case method and the daily-supply method.16 Although it is more time-consuming, the per-case method provides greater control by decreasing the amount of controlled substances that an anesthesia team member can possess; it also creates an easy audit trail.16 The daily-supply method gives anesthesia staff access to multiple quantities of narcotics that they must keep secure and return at the end of the day.16
Controlled-substance reconciliation and disposal oversight constitute a large part of the OR pharmacist’s daily responsibilities. The ASHP guidelines recommend three processes for controlled-substance documentation and waste: correlate documented administration quantities with dispensed quantities; analyze the anesthesia record to confirm use and ensure congruent documentation; and verify unadulterated drug in partially used containers.16 The disposal of waste or partially used medications should be performed in the OR satellite pharmacy by a pharmacy staff member in the presence of a witness.16 Waste can be tested with a handheld refractometer or an ultraviolet spectrometer. Refractometers are more common in OR satellites, mainly because spectrometers are more costly.17 If the OR satellite pharmacy is not open 24 hours a day, systems that closely follow the controlled-substance diversion processes performed during hours of operation must be in place.16 For example, the automated dispensing cabinet is a valuable tool for securing and recording narcotic distribution and waste after hours.
Conclusion
The advantages of having an OR satellite pharmacy are numerous. The success of an OR satellite pharmacy depends largely on the interdisciplinary relationship developed with other OR personnel. The number of OR pharmacists nationwide is limited in comparison with other hospital pharmacy positions, and these pharmacists must acquire knowledge of medication usage not routinely taught in pharmacy school. Individuals who value teamwork, possess effective problem-solving skills, and are eager to learn make excellent candidates for an OR pharmacist position.
REFERENCES
1. Feldman LS, Costa LL, Feroli ER Jr, et al. Nurse-pharmacist collaboration on medication reconciliation prevents potential harm. J Hosp Med. 2012;7:396-401.
2. Satta G, Hill V, Lanzman M, Balakrishnan I. B-lactam allergy: clinical implications and costs. Clin Mol Allergy. 2013;11:2.
3. Robinson JL, Hameed T, Carr S. Practical aspects of choosing an antibiotic for patients with a reported allergy to an antibiotic. Clin Infect Dis. 2002;35:26-31.
4. The Joint Commission. Surgical Care Improvement Project. www.jointcommission.org/surgical_care_improvement_project. Accessed January 25, 2015.
5. The Joint Commission. Specifications Manual for Joint Commission National Quality Core Measures (2010A1): Surgical Care Improvement Project (SCIP). https://manual.jointcommission.org/releases/archive/TJC2010B/SurgicalCareImprovementProject.html. Accessed January 25, 2015.
6. The Joint Commission. Specifications Manual for Joint Commission National Quality Core Measures (2010A1): Surgical Care Improvement Project (SCIP): SCIP-inf-1. https://manual.jointcommission.org/releases/archive/TJC2010B/MIF0110.html. Accessed January 25, 2015.
7. Stahel PF, Mehler PS, Clarke TJ, Varnell J. The 5th anniversary of the “Universal Protocol”: pitfalls and pearls revisited. Patient Saf Surg. 2009;3:14.
8. The Joint Commission. Specifications Manual for Joint Commission National Quality Core Measures (2010A1): Surgical Care Improvement Project: SCIP-inf-2. https://manual.jointcommission.org/releases/archive/TJC2010B/MIF0111.html. Accessed January 25, 2015.
9. The Joint Commission. Specifications Manual for Joint Commission National Quality Core Measures (2010A1): prophylactic antibiotic regimen selection for surgery. https://manual.jointcommission.org/releases/archive/TJC2010B/ProphylacticAntibioticRegimenSelectionForSurgery.html. Accessed January 25, 2015.
10. The Joint Commission. Specifications Manual for Joint Commission National Quality Core Measures (2010A1): Surgical Care Improvement Project: SCIP-inf-3. https://manual.jointcommission.org/releases/archive/TJC2010B/MIF0112.html. Accessed January 25, 2015.
11. American Society of Anesthesiologists. Statement of the labeling of pharmaceuticals for use in anesthesiology. www.asahq.org/.../Labeling Pharmaceuticals for Use in Anesthesiology/en/1. Accessed December 23, 2014.
12. PharMEDium. O.R. anesthesia. www.pharmedium.com/compounding/line/1/OR_anesthesia/OR-anesthesia-syringes.html. Accessed December 23, 2014.
13. One & Only Campaign. Safe injection practices. www.oneandonlycampaign.org/safe_injection_practices. Accessed December 23, 2014.
14. One & Only Campaign. About the campaign. http://oneandonlycampaign.org/about/the-campaign. Accessed January 25, 2015.
15. One & Only Campaign. What are safe injection practices? http://oneandonlycampaign.org/content/what-are-they-why-follow-them. Accessed January 25, 2015.
16. American Society of Health-System Pharmacists. ASHP guidelines on surgery and anesthesiology pharmaceutical services. Am J Health Syst Pharm. 1999;56:887-895.
17. Saver C. Drug diversion in the OR: how can you keep it from happening? OR Manager. 2009;25:1,8-11.
To comment on this article, contact rdavidson@uspharmacist.com.






