US Pharm. 2011;36(7):56-59.
There have been a number of articles in the past few months discussing REMS, an initiative of the FDA to control drug safety issues. REMS stands for Risk Evaluation and Mitigation Strategy and is intended to manage known or potential problems for drugs that present an especially important risk.
NDA Options
In the past, when the FDA received a new drug application (NDA) for approval, the FDA experts panel would review the drug safety and efficacy data collected by the manufacturer and either approve or deny the NDA. This up-or-down approach to voting could be very costly to manufacturers and innovators of new drugs and could prevent the marketing of drugs that have known adverse reactions but are still useful in treating specific conditions in certain circumstances.
Black Box Warnings
This concern led to a new strategy—the black box warning. The black box warning procedures were started in 2004. In 2005, the FDA mandated that all antidepressants contain a black box at the top of the labeling indicating that there could be an increased risk of suicidal tendencies in children and adolescents, later adding young adults.1 In many cases, before or after marketing of the new drug, the FDA could demand that manufacturers include the famous black box warnings about particular problems.2 This warning has also been referred to as a “black label warning” and a “boxed warning.” The black box warning has to accompany any labeling or product insert for prescription-only drugs that could cause serious side effects. At the time, this was the strongest form of warning that the FDA could issue.
Black Box Shortcomings
Apparently, the black box warnings have not been as effective as the FDA had hoped when it started this practice.3 The use of drugs with black box warnings may have waned somewhat but not to the degree the FDA thought would occur. Either prescribers do not discuss the black box warnings with patients or patients elect to take the risk of what could be severe and/or life-threatening adverse reactions.4 A listing of the drugs required to contain black box warnings is available from several online sources.5
Next Development
REMS presents a novel idea: Let the manufacturer market the otherwise risky drug but do so with restrictions. For pharmacists, the restrictions on who may dispense and administer a REMS drug are going to be the most controversial. For example, the drug rosiglitazone and its various forms (Avandia, Avandamet, and Avandaryl;
GlaxoSmithKline) are designated as REMS medications, and will not be available from all pharmacies after November 18, 2011.6
Health care providers, including physician prescribers and pharmacists along with their patients, will have to enroll in a special program established for this medication. Furthermore, patients will receive their drugs through the mail from specially certified pharmacies. “FDA’s decision to restrict access to rosiglitazone medications was made on September 23, 2010, following analysis of data suggesting an elevated risk of cardiovascular events, such as heart attack and stroke, in type 2 diabetes patients treated with the drugs. The FDA is taking this [regulatory] action to protect patients, after a careful effort to weigh benefits and risks,” said FDA Commissioner Margaret A. Hamburg, MD. “We are seeking to strike the right balance to support clinical care.”7 As might be expected, various pharmacy trade and professional groups are protesting the restricted access.
Using statistics available from the FDA, it is estimated that about 460,000 patients had prescriptions filled at outpatient retail pharmacies for rosiglitazone-containing products during the first 10 months of 2010. The agency said that about half as many patients filled prescriptions in October as had done so in January.8
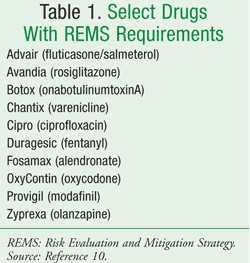
The FDA Amendments Act of 2007 gave the FDA the authority to require a REMS from manufacturers to ensure that the benefits of a drug or biologic product outweigh its risks.9 A listing of all drugs subject to the REMS regulations is also available online (TABLE 1).10 The goal of the REMS regulations is to manage a known or potential serious risk associated with a drug or biologic product to improve patient safety.11 The REMS program is actually a redefined agenda that was initially published by the FDA in 2005 in the Guidance for Industry. Development and Use of Risk Minimization Action Plans.12 This plan was also known as RiskMAPs. It differs in that REMS emphasizes an increased focus on drug safety and postmarketing reports of serious issues with the use of a drug.
New Duty for Pharmacists?
The REMS initiative begs the question of how it will impact the profession and practice of pharmacists. Will this program require pharmacists to be more vigilant in dispensing and monitoring patients taking REMS medications? Will REMS be used as another source supporting the duty of pharmacists to warn patients and/or prescribers of REMS drugs or interactions with other medications? Perhaps more concerning is whether REMS will result in more civil liability or malpractice cases against pharmacists when a patient suffers a foreseeable risk identified by the REMS program.
Speaking to a group of health care providers and manufacturers on the REMS for long-acting and extended-release opioid products in April 2011, Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research (CDER), said, “REMS is focused on the prescribers, on the patients,” and that the program “will not put new responsibilities on pharmacists.”13 At the same time, she said, “We feel that prescribers are really critical in managing this. They really have to be able to determine the pain needs of the given patient.”
However, this does not mean that pharmacists will not be involved with REMS mandates. The no “new responsibilities on pharmacists” language is misleading. The FDA does not concern itself with litigation or liability against pharmacists or pharmacies when a patient claims to have been harmed by a drug. It does not address the admissibility of literature or other testimony that a pharmacist failed to warn of the known REMS risks in a civil suit. Nor does it address the educational responsibilities that might be placed on the shoulders of pharmacists to instruct physicians and patients prescribing or taking REMS drugs. It is also silent on the question of whether a pharmacist who does not pass along REMS information about a drug to a physician or patient might result in administrative sanctions, such as the suspension or revocation of licenses by a state Board of Pharmacy.
REMS Intent
In a letter from the FDA to drug manufacturers, the agency stated that REMS will involve14:
- A medication guide for patients
- Training of health care providers who prescribe the product
- Information that prescribers can use to educate patients in the safe use, storage, and disposal of opioid-containing products
- Announcements to prescribers about the existence of the REMS and their need to complete the training
- Assessments, evaluations, and surveillance
- Instructions for companies to develop a “single, shared system.”
Pharmacy Impact
In terms of impact on the profession of pharmacy, pharmacies will be expected to provide the patient with medication guides at the very least. JoAnn Stubbings, manager of research and public policy for the ambulatory care pharmacy department at the University of Illinois at Chicago, said, “In the broadest sense, it means that pharmacists, being health care providers, should be educated on the appropriate prescribing and dispensing and counseling of long-acting opioids, as do all other health care providers.”15 Pharmacists may also be especially competent and easily accessible with respect to education of physicians and patients about safety concerns. There is also an issue as to the duty of pharmacists to report significant adverse reactions for all drugs, including those designated as REMS products. Perhaps this reporting duty, if one were to argue that it does require reports from pharmacists, will be made easier with the creation of RxEvent.org, a mechanism for reporting adverse events directly through electronic communications.16
Addressing Drug Abuse
One of the primary concerns pushing the wider use of REMS is addressing the issue of ever-increasing abuse of prescription drugs, especially opioids. FDA Commissioner Hamburg has said that REMS will “support and act in concert with” the Obama administration’s new Prescription Drug Abuse Prevention Plan.17 The FDA has stated that “opioids are at the center of a major public health crisis of addiction, misuse, abuse, overdose, and death and that it is taking action to protect patients from serious harm due to these drugs. This action represents a careful balance between continued access to these necessary medications and stronger measures to reduce their risks.”18
In May 2011, the FDA met with the Industry Working Group (IWG) and other sponsors of long-acting and extended-release opioid drugs to discuss the next steps in implementing a standard REMS system for these products, including prescriber training, medication guides, REMS assessment plans, and administrative requirements.19 The meeting resulted in FDA publication of Preliminary Responses to Industry Questions About Opioid REMS.20 This document is worth reading as it contains very specific information about how opioid REMS are to be developed and distributed. As noted before, pharmacists will be expected to furnish the patient medication guides that are required by REMS. All affected manufacturers must have their compliance plans submitted to the FDA by September 2011.
War on Drugs
This comes at a time when the so-called “War on Drugs” has been almost uniformly declared as a loss, or at the least, ineffective.21 Some commentators who have studied the global war on drugs have suggested a cease fire.22 Much how alcohol prohibition some 80 years ago did not work and created more problems than were initially foreseeable, efforts to curb the abuse of addictive or dependence-producing drugs has also resulted in unexpected consequences. Angelo Codevilla of the Claremont Institute, who has studied efforts to alter drug-use behavior, evoked the Pogo cartoon cliché “we have met the enemy and it is us” when speaking about the importation of illegal drugs from Mexico: “The border is unfriendly not because of too few fences, drones, or soldiers, but because American drug habits finance the traffickers. These dollars, and nothing else, are responsible for the near collapse of law and order south of the border and for the insufficiently publicized corruption on the northern side.”23
Internet and Other Sources for Drugs of Abuse
Lest we think Mexico is the source of all evils when it comes to smuggling illegal drugs, a recent story in The Economist reported that Canada now trumps Mexico as an entryway into the U.S. for the drug ecstasy.24 Complicating matters even more, Internet orders for drugs is increasing the abuse rate in this country.25 Investigators from Massachusetts General Hospital and the University of Southern California found that over a 7-year period, states with the greatest expansion in high-speed Internet access also had the largest increase in admissions for treatment of prescription drug abuse. “Our findings suggest that Internet growth may partly explain the increase in prescription drug abuse, since it is well known that these drugs are easily available online,” said Dana Goldman, director of the Schaeffer Center for Health Policy and Economics at the University of Southern California.26 She went on to state that their findings show that prescription drugs are fast replacing illegal substances in many areas, but especially on college campuses.
Analysis
The development of REMS regulations comes at a time when prescription drug abuse is outpacing the use of illegal drugs (e.g., heroin, LSD). The initiative is an attempt to control not only drugs of abuse but also risks associated with drugs that are not often abused. Will REMS cure drug abuse or lower the incidence of major risks with other drugs? It is much too early to draw any conclusions. One thing is certain—pharmacists will incur extra dispensing duties with more and more drugs having mandatory patient medication guides. REMS is also a source of additional liability for pharmacists when something goes wrong. The impact of REMS on the profession of pharmacy is certainly much greater than that portrayed by the FDA. At the very least, REMS will provide an opportunity for pharmacists to gain more knowledge about potential dangers associated with these drugs and turn that information into a source of education for prescribers and patients alike.
REFERENCES
1. FDA proposes new warnings about suicidal thinking, behavior in young adults who take
antidepressant medications. FDA news release. May 2, 2007. www.fda.gov/NewsEvents/
2. FDA. Guidance for industry. Warnings and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products—content and format. January 2006. www.fda.gov/downloads/Drugs/
3. Shah ND, Montori VM, Krumholz HM, et al. Responding to an FDA warning—geographic variation in the use of rosiglitazone. N Engl J Med. 2010;22:2081-2084.
4. Cohen A, Rabbani A, Shah N, Alexander GC. Changes in glitazone use among office-based physicians in the United States, 2003-2009. Diabetes Care. 2010;33:823-825.
5. See, e.g., Black Box Rx. http://blackboxrx.com/. Accessed June 14, 2010.
6. REMS now available for Avandia; access to drug restricted. Modern Medicine Formulary. June 3, 2011. http://formularyjournal.
7. See Note 6, supra.
8. Traynor K. Rosiglitazone REMS arrives in November (2011). ASHP Pharmacy News. June 13, 2011. www.ashp.org/menu/News/
9. FDA. Approved Risk Evaluation and Mitigation Strategies (REMS). www.fda.gov/Drugs/DrugSafety/
10. See Note 9, supra. See also: FDA. List of long-acting and extended-release opioid products required to have an opioid REMS. www.fda.gov/Drugs/DrugSafety/
11. Shah M. REMS update: solutions for REMS implementation in hospital and retail settings. Drug Topics. May 15, 2011. http://drugtopics.
12. FDA. Guidance for industry. Development and use of risk minimization action plans. March 2005. www.fda.gov/downloads/Drugs/
13. Thompson CA. No new pharmacist responsibilities with upcoming opioid REMS. ASHP Pharmacy News. April 19, 2011. www.ashp.org/menu/News/
14. See Note 13, supra.
15. See Note 13, supra.
16. Network for reporting adverse drug events goes live. Healthcare IT News. June 14, 2011. www.healthcareitnews.com/news/
17. See Note 13, supra.
18. FDA. Opioid drugs and Risk Evaluation and Mitigation Strategies (REMS). May 11, 2011. www.fda.gov/drugs/drugsafety/
19. See Note 18, supra.
20. FDA. Preliminary responses to industry questions about opioid REMS. www.fda.gov/Drugs/DrugSafety/
21. War on Drugs: Report of the Global Commission on Drug Policy. June 2011. www.globalcommissionondrugs.
22. O’Grady MA. More calls for a drug war cease-fire. Wall Street Journal. June 6, 2011. http://online.wsj.com/article/
23. See Note 22, supra.
24. See Note 3, supra. See also: The drug trade in North America. Ecstatic traffickers. The Economist. May 23, 2011. www.economist.com/blogs/
25. Kransy R. Internet tied to growth in U.S. prescription drug abuse. May 12, 2011. Modern Medicine. www.reuters.com/article/2011/
26. See Note 25, supra.
To comment on this article, contact rdavidson@uspharmacist.com.





