US Pharm. 2009;34(10):10.
The FDA may request that a sponsor seeking approval of a new drug conduct a postmarketing study. Such surveillance is commonly known as a postmarket commitment (PMC). PMCs, identified during the application review process, do not represent major unaddressed safety and efficacy concerns, but rather are intended to further establish the safety, efficacy, or optimal use of a product or ensure that a product's quality is consistent and reliable.
PMC by Application Type: Between 2002 and 2005, 245 original drug product and supplemental applications were approved with agreed-upon PMCs. These applications, including 184 new drug applications (NDAs) and 61 biologic license applications (BLAs), contained 743 PMCs. Thirty percent of applications (48% of original NDAs, 27% of NDA supplements, 13% of original BLAs, and 12% of BLA supplements) had at least one PMC. Nearly half of original NDAs and all original BLAs were classified as new molecular entities (NMEs). According to the FDA's 2008 Evaluations and Initiatives, the number of PMCs per application was more broadly distributed for NME applications than for non-NME applications.
Reasons for PMC: PMCs were most often requested based on a need for additional data, e.g., safety signals, underrepresented subpopulations, and drug-drug interaction (DDI) concerns. The most common reason (in 33% of PMCs) for requesting a PMC rather than requiring a completed study for approval was that the issue required further definition, but did not affect determination of safety and efficacy. Other reasons were that the issue was a theoretical concern and that long-term data were required.
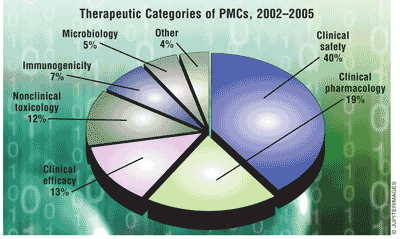
Types of PMCs: Clinical safety studies were more common in BLAs than in NDAs, and more common in non-NME applications than in NME applications. Nonclinical toxicology PMCs were more common in NME applications than in non-NME applications, and much more common in NDAs than in BLAs. Microbiology studies were limited to antimicrobial NDAs, and almost all immunogenicity studies occurred in BLAs.
Timeliness: Sponsors submitted protocols and final study reports by the milestone date 76% and 60% of the time, respectively. FDA reviewers met their goal dates for completing annual status report reviews 53% of the time, and final study report reviews 61% of the time. Thirty-four percent of PMCs were completed and 66% remained open; of open PMCs, 81% were progressing on schedule. The most common reason for delayed PMC status was difficulty with clinical trial enrollment (29%).
Results: Fifty-one percent of fulfilled PMCs resulted in a label change. The most common reasons for a label change were validated safety and efficacy concerns, validated DDI concerns, and expanded use in subpopulations.
To comment on this article, contact rdavidson@jobson.com.





