US Pharm. 2010;35(5)(Diabetes suppl):8-13.
Diabetes is a complex metabolic disorder characterized by hyperglycemia that occurs from defects in insulin secretion, insulin action, or both. Approximately 95% of patients diagnosed with diabetes will have type 2 diabetes.1 Type 2 diabetes occurs from progressive deterioration of insulin secretion from pancreatic beta cells coupled with insulin resistance. Most patients with type 2 diabetes are also obese, which further perpetuates insulin resistance. Furthermore, the chronic state of hyperglycemia worsens the continuous decline of beta-cell function.1,2
In 2007, the estimated prevalence of diabetes was 23.6 million people in the United States alone.3 Approximately 10% of non-Hispanic whites have diabetes, and in non-Hispanic blacks the prevalence exceeds 14%. In 2006, diabetes was the seventh leading cause of death, and it remains the leading cause of new cases of blindness and kidney failure. Diabetes is also responsible for approximately 60% of lower limb amputations. For every point drop in hemoglobin A1C (HbA1C) patients can reduce their risk for microvascular complications by 40%.3 Unfortunately, approximately 36% of patients do not reach the goal A1C of <7% on either monotherapy or combination therapy.4 Therefore, glycemic control is crucial to diabetes management and the reduction of diabetes-related complications.
Diagnosis
Diabetes can be diagnosed using one of the following methods: HbA1C ≥6.5%; fasting plasma glucose ≥126 mg/dL; 2-hour plasma glucose ≥200 mg/dL following an oral glucose tolerance test using 75 g of anhydrous glucose dissolved in water; or a random plasma glucose ≥200 mg/dL in patients with classic symptoms of hyperglycemia, such as polyuria, polyphagia, and polydipsia (TABLE 1). The first three criteria should be confirmed with repeat testing before a diagnosis of diabetes can be determined. Glycemic control is defined by an A1C of <7%.5
Many available pharmacologic interventions effectively manage diabetes and help patients to achieve their glycemic goal. Several agents, however, are limited by side effects, such as weight gain and hypoglycemia. Incretin-based therapies represent a newer class of agents effective in lowering HbA1C without the side effects of hypoglycemia and weight gain.6,7

The “Incretin Effect”
The incretin concept was identified from the difference in insulin response to an oral glucose load compared to an IV glucose load.6 The “incretin effect” can be defined by the greater insulin response to an oral glucose load than that experienced with an IV glucose challenge.4,6,8 There are two incretin hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1). GIP, derived from purified porcine intestinal substance, was identified first and determined to have minimal effects on gastric acid secretion and a significant impact on insulin response in humans. GIP is produced from the duodenal and jejunal enteroendocrine K cells of the small bowel. GLP-1, synthesized from the enteroendocrine L cells of the distal ileum and colon, resulted from the cloning of complementary DNAs (cDNAs) and genes that encoded proglucagon. GLP-1 exists as GLP-1(7-37) and GLP-1(7-36), with GLP-1(7-36) being more prevalent after food intake.8 Both hormones, GIP and GLP-1, are secreted within minutes of the presence of food; however, GLP-1 is critical for glucose control.8
While both GIP and GLP-1 promote beta-cell survival through cell proliferation at the site of G-protein-coupled receptor activation, GLP-1 is associated with additional glucose-lowering benefits. GLP-1 improves defective glucose-stimulated insulin secretion, slows insulin secretory response to meals, inhibits the secretion of glucagon, and increases the synthesis of proinsulin in the pancreatic beta cell. These effects are important to the pancreatic beta cell, whose progressive loss of cell mass and decline in function are characteristic of type 2 diabetes.4 The time that incretin hormones are available in the circulation is, however, minimal.
Circulating GIP and GLP-1 are rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4) and renal clearance. DPP-4 is an aminopeptidase found in the liver, lungs, kidneys, intestinal brush-border membranes, lymphocytes, and endothelial cells.6,8 DPP-4 is essential to incretin inactivation, allowing only 10% to 20% of GLP-1 to be biologically active with a half-life of less than 2 minutes. The inhibition of DPP-4 produces actions similar to that of GLP-1; i.e., stimulation of insulin secretion, glucagon inhibition, and beta-cell mass preservation.8 Therefore, newer diabetes agents have focused therapy development on creating GLP-1 analogs that are resistant to DPP-4 or agents that will inhibit DPP-4.4
In type 2 diabetes, the incretin effect is diminished or lost. It is suspected that the defect occurs because of a weakened action of GIP and the significant decrease of circulating GLP-1 after food ingestion, resulting from diminished secretion of GLP-1.9,10 Antidiabetic agents that mimic or prolong the action of GLP-1 are therefore important for glucose control.8
There are multiple therapies available for the management of diabetes. Traditional therapies manage type 2 diabetes by increasing insulin secretion; decreasing insulin resistance by reducing lipotoxicity; decreasing the hepatic output of glucose; or blocking the absorption of glucose in the gastrointestinal (GI) tract.4 Side effects of therapy (e.g., hypoglycemia, weight gain, and GI discomfort) are challenges associated with traditional pharmacologic agents and may be related to poor medication adherence and thus poorer patient outcomes. Newer agents seek to improve glycemic control while minimizing troublesome side effects.11
The two classes of medications currently available to target the incretin system are incretin mimetics/enhancers and DPP-4 inhibitors. Incretin mimetics resemble the biologic GLP-1 and are associated with transient nausea and GI discomfort. In addition, GLP-1 agonists (i.e., mimetics) are peptides and must be administered subcutaneously.9 DPP-4 inhibitors are another option to prolong the benefits of GLP-1 that also have proven efficacy, good bioavailability (i.e., 60%-90%), minimal side effects, and likely improved patient compliance from oral dosing.8,10 In general, DPP-4 inhibitors offer 0.5% to 0.8% reduction in HbA1C.12 Currently, there are two marketed DPP-4 inhibitors, sitagliptin and saxagliptin.
Dipeptidyl Peptidase-4 Inhibitors
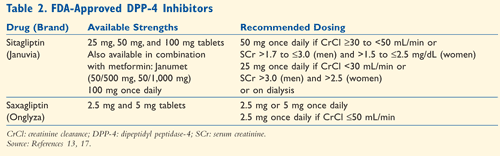
Sitagliptin: The first DPP-4 inhibitor to enter the U.S. market, Sitagliptin (Januvia), was approved in October 2006 as adjunctive therapy to diet and exercise in the treatment of type 2 diabetes.13 It is a once-daily, highly selective, potent inhibitor of DPP-4 enzymes. Sitagliptin is eliminated primarily by the kidneys, with approximately 79% of the drug unchanged in patients with normal renal function.14 The dose must be adjusted in patients with both moderate and severe renal impairment (TABLE 2). Sitagliptin is not an inhibitor of cytochrome P isozymes or an inducer of CYP3A4, so drug interactions are minimal.
Phase III trials of sitagliptin revealed that it was well tolerated at doses of 100 mg once daily, either alone or in combination.11 Sitagliptin was studied in participants with an average A1C of 8% in combination with metformin at doses >1,500 mg/day. After 24 weeks, more patients achieved goal A1C <7% in the sitagliptin group (47%), with only 18.3% in the metformin alone group. The reduction in A1C from baseline was also statistically significant in the sitagliptin group (P <.001). Only 4.5% of participants required rescue therapy with pioglitazone for inadequate glycemic control (P <.001).15
Despite proven efficacy, there are recent safety concerns regarding sitagliptin use. In a 24-week study, patients were randomized to receive either glimepiride alone or in combination with metformin, or glimepiride and sitagliptin or both agents also in combination with metformin.16 Rescue therapy was available with pioglitazone. Groups that included sitagliptin did achieve a lower A1C from baseline compared to the placebo groups (P <.001); however, there was an increased incidence of hypoglycemia in the sitagliptin group compared to the placebo groups. Patients within both sitagliptin groups had 27 events of hypoglycemia (12.2%), with the most occurring in the group receiving both glimepiride and metformin. The placebo groups experienced four events of hypoglycemia (1.8%) (P <.001).16
It is now recommended that when using agents that promote insulin secretion, such as insulin secretagogues, or insulin in combination with sitagliptin, those doses be reduced.12 Furthermore, postmarketing reports of acute pancreatitis, both fatal and nonfatal hemorrhagic and necrotizing, and rare hypersensitivity reactions such as angioedema and Stevens-Johnson syndrome may limit use in certain populations.13 The hypersensitivity reactions usually occurred within the first 3 months of therapy. Sitagliptin is contraindicated in patients with a history of hypersensitivity reactions to sitagliptin.13
Saxagliptin: Saxagliptin (Onglyza) was approved by the FDA in July 2009. Like sitagliptin, saxagliptin is also indicated as an adjunct to diet and exercise for the management of type 2 diabetes in adults.17 Saxagliptin is a reversible, potent inhibitor that is selective for DPP-4 by more than 4,000-fold, compared with sitagliptin, whose selectivity is more than 2,600-fold.6,18,19 Similar to sitagliptin, saxagliptin is excreted by the kidneys, but it also undergoes hepatic metabolism. Dosage adjustments are only required in patients with severe renal insufficiency.11 Saxagliptin is metabolized by CYP isozymes, resulting in an increase in the concentration of saxagliptin when used in combination with 3A4 inhibitors. Therefore, a dosage reduction to 2.5 mg is required. Common adverse reactions include headache, upper respiratory infection, and urinary tract infection.17
Saxagliptin has been studied both alone and in combination with metformin, pioglitazone, and glyburide. The efficacy of saxagliptin as add-on therapy with metformin was evaluated in 743 patients with an A1C between 7% and 10% for a 24-week period, compared to metformin alone.20 Participants were randomized to saxagliptin groups at 2.5 mg, 5 mg, and 10 mg in combination with metformin doses ranging from 500 to 2,550 mg/day at study entry. At 24 weeks, a statistically significant reduction in A1C occurred in all treatment groups (P £.0001), compared to the metformin plus placebo group. Also in each treatment group, a significant number of participants achieved a goal A1C <7% at 37.1%, 43.5%, and 44.4% in saxagliptin/metformin groups 2.5 mg, 5 mg, and 10 mg, respectively (P £.0001).20
Similar results were obtained in a phase III trial including saxagliptin 5 mg and 10 mg in combination with maximal doses of metformin compared to saxagliptin and metformin alone. The participants in this study had a higher A1C (8%-12%). At 24 weeks, the combination groups had a statistically significant reduction in A1C (-2.5% and -2.5% vs. -1.7% and 2.0%; P <.0001).20
Advantages and Disadvantages to DPP-4 Inhibitor Use
DPP-4 inhibitors offer the option for improvement in both HbA1C and beta-cell survival. In addition, risks associated with hypoglycemia are minimal and usually only occur in combination therapy with insulin secretagogues or insulin. Weight gain is also of concern in diabetes patients. Though DPP-4 inhibitors do not promote weight loss, they are weight neutral and can prevent further weight gain. Other therapies that target the incretin system cause GI discomfort and may worsen patient compliance. This effect does not occur within the DPP-4 inhibitor class of agents. Finally, convenient once-daily oral dosing may promote patient adherence and improve health outcomes.8
Although DPP-4 inhibitors have proven to be efficacious in reducing HbA1C and improving beta-cell function, long-term clinical trial data are not yet available to assess the sustainability of glycemic control and protection of beta-cell mass.4,8,10 With several DPP-4 inhibitors in clinical development, their long-term safety profile as well as cardiovascular morbidity and mortality has become of significant interest. Therefore, further long-term studies with cardiovascular end points are warranted. Each agent is unique, however, and will likely differ in adverse effects. Additional concerns surround DPP-4 inhibitors and their interference with immune function. That too is poorly understood and warrants further research.12
Place in Therapy
The American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) developed an algorithm to assist providers in managing type 2 diabetes. When developing the algorithm, the AACE and ACE identified minimizing hypoglycemia and weight gain as their top priorities in drug therapy selection. As a result of that, initial recommendations focus on the use of metformin or a thiazolidinedione if metformin is contraindicated, followed by a GLP-1 agonist or a DPP-4 inhibitor.11
In January 2009, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) presented a medical management consensus statement that included a treatment algorithm to manage diabetes somewhat different from that of the AACE and ACE.12 The algorithm is divided into two tiers and suggests a stepwise treatment approach. Tier 1 is well-validated core therapies consisting of lifestyle interventions, metformin, insulin, and second-generation sulfonylureas. Tier 2 is less well-validated therapies and includes agents from tier 1 with the addition of pioglitazone and GLP-1 agonists. DPP-4 inhibitors were not included in the two tiers because their effectiveness on glycemic control is lower than or equivalent to first- and second-tier agents, as well as for less clinical evidence and relative expense. The consensus statement does suggest, however, that DPP-4 inhibitors may be appropriate in select populations based on concerns associated with weight gain and hypoglycemia, similar to the AACE and ACE recommendations.12
Summary
DPP-4 inhibitors offer improved glycemic control in the management of type 2 diabetes, both alone and in combination with other antihyperglycemic agents, by reducing HbA1C and improving beta-cell function. Agents such as sitagliptin and saxagliptin are available for first-line or adjunctive therapy. DPP-4 inhibitors have an attractive side effect profile that encourages the prevention of weight gain without hypoglycemia, making this class of agents an option for early intervention in the management of type 2 diabetes.
REFERENCES
1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62-S69.
2. Gallwitz B. Saxagliptin, a dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. IDrugs. 2008;11:906-917.
3. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. CDC. www.cdc.gov/diabetes/pubs/pdf/
4. Crepaldi G, Carruba M, Comaschi M, et al. Dipeptidyl peptidase 4 (DPP-4) inhibitors and their role in type 2 diabetes management. J Endocrinol Invest. 2007;30:610-614.
5. American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(suppl 1):S11-S61.
6. Tahrani AA, Piya MK, Barnett AH. Saxagliptin: a new DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Adv Ther. 2009;26:249-262.
7. Chacra AR, Tan GH, Apanovitch A, et al. Saxagliptin added to submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract. 2009;63:1395-1406.
8. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696-1705.
9. Deacon CF. Incretin-based treatment of type 2 diabetes: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2007;9(suppl 1):23-31.
10. Halimi S. DPP-4 inhibitors and GLP-1 analogues: for whom? Which place for incretins in the management of type 2 diabetic patients? Diabetes Metab. 2008;34(suppl):S91-S95.
11. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an AACE/ACE consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15:540-549.
12. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
13. Januvia (sitagliptin) package insert. Whitehouse Station, NJ: Merck & Co., Inc; February 2010.
14. Bergman AJ, Cote J, Yi B, et al. Effects of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care. 2007;30:1862-1864.
15. Charbonnel B, Karasik A, Liu J, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638-2643.
16. Hermansen K, Kipnes M, Luo E, et al. Efficacy and safety of dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733-745.
17. Onglyza (saxagliptin) package insert. Princeton, NJ: Bristol-Myers Squibb; July 2009.
18. Rosenstock J, Aguilar-Salinas C, Klein E, et al. Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin. 2009;25:2401-2411.
19. Jadzinsky M, Pfützner A, Paz-Pacheco E, et al. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab. 2009;11:611-622.
20. DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32:1649-1655.
To comment on this article, contact rdavidson@uspharmacist.com.





