US Pharm. 2010;35(6)(Generic Drug Review):30-35.
Graves’ disease is an autoimmune disorder affecting approximately 0.3% of people living in the United States and 0.5% of the world population.1 Graves’ disease may include thyroid enlargement, ophthalmopathy, dermopathy, and hyperthyroidism. The most common cause of hyperthyroidism, this disorder occurs five to 10 times more often in women than in men, usually developing between the ages of 20 and 50 years.1,2 While the etiology of Graves’ disease is unknown, hereditary genetics has been implicated as predisposing an individual to developing the disorder later in life through environmental factors, such as smoking and stressful life events, or hormonal triggers like childbirth.2,3 This article will concentrate on the clinical presentation of Graves’ disease and currently available treatment options.
Thyroid Function in Graves’ Disease
Normally, the thyroid gland synthesizes, stores, and releases two kinds of hormones: thyroxine (T4) and triiodothyronine (T3). Iodine obtained through dietary intake is absorbed via the gastrointestinal (GI) tract; it is used as iodide by the thyroid follicular cells and converted to either monoiodotyrosine (MIT) or diiodotyrosine (DIT). MIT and DIT undergo coupling to form T3; T4 is formed by coupling of two DITs. The hypothalamic-pituitary negative feedback system signals the thyroid to stop hormone release when serum T4 and T3 levels are too high.1,4 In Graves’ disease, however, the negative feedback system is superseded by thyroid-stimulating antibodies activating thyroid receptor cells in the same way that thyroid-stimulating hormone (TSH) stimulates the thyroid gland.1,2 Autoantibody stimulation leads to thyroid enlargement by way of thyroid-cell hyperplasia and hypertrophy, along with an increase in serum T3 and T4 and suppression of TSH, leading to a thyrotoxic state.2
Clinical Presentation
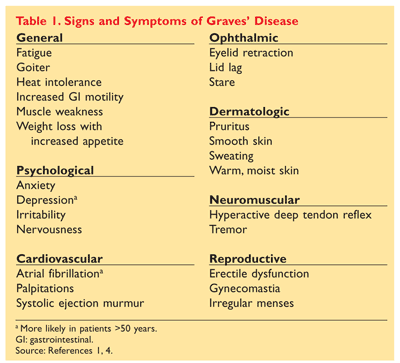
Upon physical examination, most patients exhibit an enlarged thyroid gland that is typically two to three times bigger than normal (>40 g). Extrathyroidal complications of ophthalmopathy and dermopathy also may be seen, and deep tendon reflexes usually are hyperactive.1 In a rare subset of individuals, deep tendon reflexes may be diminished as a result of hypokalemic periodic paralysis. More commonly seen in Asian and Hispanic patients, this phenomenon is related to an intracellular shift of potassium, usually after a period of strenuous exercise. Total body potassium remains normal. Periodic paralysis usually affects the group of muscles exercised and ranges from mild weakness to complete paralysis.1
Ophthalmopathy is apparent in about 30% of diagnosed individuals, with detection in more than 80% of patients assessed by orbital imaging.2,5 Photophobia is common, along with lid lag, periorbital edema, and proptosis. Other signs and symptoms include cardiovascular, central nervous system, and general mood problems, among others. See TABLE 1.1,2,4,6
Diagnosis
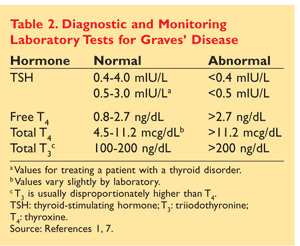
In addition to gross signs and symptoms, abnormal laboratory values are used to make a clinical diagnosis of Graves’ disease (TABLE 2).1,7 Total T3, free T4, and TSH values usually reveal increases in T3 and T4 levels, with T3 being disproportionately higher than T4. TSH levels are extremely low or nondetectable owing to negative feedback from the pituitary gland.1,4,5,7 In patients not displaying overt signs and symptoms and in those experiencing recent onset of symptoms, a 24-hour radioactive iodine (RAI) uptake may be done to determine whether the thyroid gland is producing thyroid hormone (T4, T3, or both) when it is already in a thyrotoxic state. This procedure is performed only in nonpregnant individuals. Normal RAI uptake ranges from 10% to 30%; values higher than 30% indicate Graves’ disease.1,5
Treatment
Once a diagnosis has been made, the patient’s treatment plan needs to be determined. Treatment options focus on the individual, taking into consideration the patient’s age, comorbidities, preference, and convenience. Three treatment options currently exist: thyroidectomy, antithyroid medications, and RAI.1-3,8
Thyroidectomy: A nonpharmacologic approach rarely used in the U.S. for Graves’ disease is surgical removal of the thyroid gland, which produces a euthyroid or hypothyroid state. Surgery is considered in patients who have a large goiter, have severe ophthalmopathy, are young, are pregnant or lactating, or have relapsed after a trial of antithyroid medications.1,2,4,9 With an 80% to 90% cure rate, surgical removal is considered safe and effective when performed by an experienced surgeon.1 However, the surgery comes with its own risks, including vocal-cord injury, hypoparathyroidism (1%-2%), transient hypocalcemia, bleeding, and infection. Permanent hypothyroidism is a direct result for the majority of patients who undergo surgery, and lifelong thyroid-hormone supplementation and monitoring are required.1,3,4
Antithyroid Medications: In Graves’ disease, propylthiouracil (PTU) and methimazole (MMI) are given to inhibit thyroid production. The primary objective of using antithyroid medications is to induce remission.1 Maximum remission rates of 30% to 50% are observed after 12 to 24 months of therapy. Remission is defined as a normal thyrotropin level while no medication is being taken. Patients who exhibit a smaller goiter (<50 g), are older (>40 years), have a short disease duration (<6 months), have taken antithyroid medication for 1 to 2 years, or have no history of relapse are more likely to experience remission.1-3
Both PTU and MMI are absorbed well from the GI tract, with respective half-lives of 1 to 2.5 hours and 6 to 9 hours. Peak serum concentrations are observed within 1 hour after ingestion.1,10,11 Only PTU inhibits peripheral conversion of T4 to T3; however, the effect is dose-related.1,3 Thionamides have been associated with decreasing thyroid-stimulating immunoglobulins in Graves’ disease; on the other hand, normalization of thyroid-hormone levels has shown a similar effect.3 Generally, normalization of hormone levels and symptom control occur 3 to 6 weeks after therapy is initiated. Loading doses, which range from 300 mg to 600 mg for PTU and 10 mg to 40 mg for MMI, are given for 4 to 6 weeks, followed by a dose reduction when symptoms resolve. For both PTU and MMI, the loading dose is normally given in 3 divided doses. The total daily maintenance dose ranges from 50 mg to 300 mg for PTU and 5 mg to 10 mg for MMI.1,3,6 PTU comes in 50-mg tablets and MMI is available in 5-mg and 10-mg tablets. MMI is typically taken once daily, whereas PTU is usually dosed 3 times daily.10,11
The side-effect profiles of PTU and MMI are similar. Arthralgias, rash, mild leukopenia (WBC <4,000 mm³), and urticaria occur in approximately 5% to 25% of patients taking thionamides. Arthralgias normally occur after 6 months of therapy. These effects are considered minor.1,3,8
Hepatotoxicity is a rare but severe side effect seen with both PTU and MMI. In June 2009 the FDA warned health care professionals about reports of PTU-induced liver failure.12 Currently, PTU-induced liver failure has been reported in 33 adults and 14 children. As reported by the United Network for Organ Sharing, from 1990 to 2007, 16 adults and 7 children received a liver transplant, for an average of two per year in the U.S. alone. MMI, also known for its hepatotoxicity, was not known to occur in patients receiving liver transplants during the same time period. Hepatotoxicity, reported with an average dose of 300 mg, usually occurred during the first 6 months of treatment. As a result, the American Thyroid Association (ATA) and the American Association of Clinical Endocrinologists (AACE) are drafting new hyperthyroidism guidelines that are slated to be published sometime in 2010. In the meantime, the ATA and AACE have recommended PTU as a second-line medication after MMI, except during the first trimester of pregnancy or in patients who are allergic to MMI. PTU should not be used in pediatric patients owing to their increased risk for liver damage. Patients should be encouraged to immediately report to their physician any symptoms of liver failure, such as yellowing of the eyes or skin, fatigue, malaise, pharyngitis, nausea, anorexia, or easy bruising.12,13
Agranulocytosis is a serious side effect occurring in 0.1% to 0.6% of patients taking thionamides. It is recommended that patients discontinue taking the thionamide and contact their physician if they experiencing fever, sore throat, mouth ulcers, or malaise. Onset tends to be sudden. Routine monitoring is not recommended.2,5
RAI (I131): Patients whose Graves’ disease is more severe and those who have relapsed after antithyroid therapy are candidates for RAI treatment. RAI given as I¹³¹ is the preferred drug for treating Graves’ disease in the U.S., as it is rapidly absorbed into and concentrated in thyroid follicular cells. Beta particles emitted from the absorbed I¹³¹ cause follicular-cell necrosis and destruction within weeks to years of treatment.1,4
RAI is administered in a clear liquid or tablet form. One approach to dosage is to give the patient a single fixed dose between 5 and 15 mCi; another approach is to calculate a specific dose based on age, gender, gland size, and thyroid uptake.3,14,15 Patients taking 30 mCi or more usually require hospitalization; patients taking less than 30 mCi usually are sent home and encouraged to take certain safety precautions for several days to minimize exposure to family and friends, such as staying 3 to 6 feet away from people at all times and sleeping in a separate bedroom. Precautions vary according to the dose given.3,6,14-16
Initially, the patient may get worse after treatment, as preformed thyroid hormone is released. Patients with cardiovascular complications and those who are elderly are usually prescribed thionamides, beta-blockers, or corticosteroids to manage these effects. Thionamides are generally given prior to I¹³¹ treatment to stabilize hormone production. Thionamides are stopped 4 to 6 days before treatment and then restarted approximately 4 days after therapy. Corticosteroid treatment tends to reduce T3 and T4 concentrations following RAI treatment.1,5
Normal thyroid function can occur as early as 6 to 8 weeks after treatment in 50% to 75% of patients. However, hypothyroidism results in more than 90% of patients within the first year after treatment, with a 2% to 3% rate each year thereafter.1,3,9 Lifelong thyroid supplementation and monitoring are necessary after treatment. Initially, monitoring occurs monthly; once a euthyroid state is obtained, monitoring is reduced to every 6 to 12 months. Mild thyroid-gland tenderness and dysphagia may occur. Reports of carcinomas or congenital defects are mixed; some studies report an increased risk, while others suggest no association. One study, however, reported an increased risk in all-cause mortality, including mortality from cardiovascular and cerebrovascular disease.1,2
Adjunctive Medications: Beta-blockers, calcium channel blockers, and centrally acting sympatholytics are used to control symptoms of hyperthyroidism such as anxiety, palpitations, heat intolerance, sweating, and tremors.1-3 These medications do not decrease thyroid-stimulating antibodies (TSAb) or prevent thyroid storm; propranolol and nadolol have been shown to partially inhibit conversion of T4 to T3, but the therapeutic effect is small.1,3 First-line treatment usually begins with a beta-blocker, particularly propranolol. The initial starting dose ranges from 20 mg to 40 mg four times daily, but may be up to 240 mg to 480 mg/day. Higher doses typically are used in patients who are younger or who are in a severely toxic state, as they tend to clear the drug faster.1,4 As with all beta-blockers, these medications are contraindicated in patients with decompensated heart failure, sinus bradycardia, or spontaneous hypoglycemia and in those who are taking monoamine oxidase inhibitors or tricyclic antidepressants.
More common side effects include nausea, vomiting, bradycardia, insomnia, anxiety, masked signs of hypoglycemia, and impotence. Nonselective beta-blockers should be used with caution in patients suffering from asthma or bronchospastic disorders. Calcium channel blockers such as diltiazem and centrally acting sympatholytic agents such as clonidine are used if beta-blockers are contraindicated.1,17
Iodide was the first drug used in Graves’ disease that is able to block thyroid hormone release, decreasing thyroid size and vascularity as well as inhibiting hormone synthesis. Effects may be seen as early as 2 to 7 days, but concentrations of T4 and T3 are reduced for only a few weeks. Iodides are now used to prepare patients for surgery or after RAI treatment.1,4
Potassium iodide (SSKI) or Lugol’s solution is typically used. SSKI contains between 35 and 50 mg of iodide per drop, and Lugol’s solution contains approximately 6 mg per drop. SSKI is usually given as 3 to 10 drops daily in water or juice and is administered 7 to 14 days before surgery. However, if the patient is undergoing RAI, SSKI is administered 3 to 7 days after treatment in order not to interfere with RAI being taken up into the thyroid. The most common side effects are salivary-gland swelling, hypersensitivity reactions, metallic taste, sore teeth and gums, stomach upset, diarrhea, and gynecomastia.1,4
Lithium has been used in the past to inhibit the release of thyroid hormone. The exact mechanism of action is unknown, but lithium is believed to act similarly to iodide. With its transient effect and side-effect profile, lithium has limited usefulness in the treatment of Graves’ disease.4
Treatment During Pregnancy
Hyperthyroidism is suspected during pregnancy when there is persistent tachycardia along with an inability to gain weight despite a good appetite. No increase in maternal morbidity and mortality is seen. However, around 20% of patients experience thyroid storm during the postpartum period if left untreated. Other side effects, such as low fetal birthweight, premature delivery, fetal loss, and pre-eclampsia, may occur if the disease is left untreated. Fetal and neonatal hyperthyroidism may occur owing to TSAb crossing the placenta.1,3
RAI is contraindicated during pregnancy, and surgery is considered a second-line alternative. The treatment of choice is antithyroid medication. In the past, MMI was not recommended during pregnancy owing to rare complications like fetal aplasia cutis and fetal GI problems. However, both PTU and MMI have been shown to cross the placenta and may interact with the developing fetus, especially at higher doses. Lower doses are recommended, with PTU still being the preferred drug during the first trimester.1,8 The typical PTU dose is 300 mg or less daily to prevent fetal goiter or hypothyroidism. Normally, PTU is tapered to 50 mg to 150 mg daily after the first 4 to 6 weeks of treatment. Free T4 is usually kept near the upper limit of normal during pregnancy. Some patients go into remission during the last trimester, as TSAb tend to fall. Postpartum hyperthyroidism occurs in approximately 10% of women, however.1,3
Summary
Graves’ disease is an autoimmune disorder that affects millions of people worldwide. A patient who is experiencing weight loss, dermopathy, ophthalmopathy, and/or cardiovascular complications needs to be referred to his or her primary care physician for thyroid-function tests. Diagnosis lies in overt signs and symptoms in combination with abnormal laboratory tests. There are currently three main treatment strategies, with RAI preferred during relapse and more severe disease, and antithyroid medications preferred during pregnancy. It is important for patients to have lifelong thyroid-function monitoring, as levels vary with age, during pregnancy, and even with the different treatment options.
REFERENCES
1. Sherman SI, Talbert RL. Thyroid disorders. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 7th ed. New York, NY: McGraw-Hill Medical; 2008:1243-1263.
2. Brent GA. Graves’ disease. N Engl J Med. 2008;358:2594-2605.
3. Hegedüs L. Treatment of Graves’ hyperthyroidism: evidence-based and emerging modalities. Endocrinol Metab Clin N Am. 2009;38:355-371.
4. Streetman DD, Khanderia U. Diagnosis and treatment of Graves disease. Ann Pharmacother.
5. Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ. 2006;332:1369-1373.
6. Iagaru A, McDougall IR. Treatment of thyrotoxicosis. J Nucl Med. 2007;48:379-389.
7. MedlinePlus. TSH. www.nlm.nih.gov/medlineplus/ency/article/003684.htm. Accessed March 9, 2010.
8. Cooper DS. Antithyroid drugs. N Engl J Med. 2005;352:905-917.
9. Stålberg P, Svensson A, Hessman O, et al. Surgical treatment of Graves’ disease: evidence-based approach. World J Surg. 2008;32:1269-1277.
10. Tapazole (methimazole) package insert. Bristol, TN: King Pharmaceuticals Inc; February 2009.
11. Propylthiouracil package insert. Pearl River, NY: Lederle; 2000.
12. FDA. Drug Safety and Availability. Information for healthcare professionals—propylthiouracil-induced liver failure. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm162701.htm. Accessed March 9, 2010.
13. Cooper DS, Rivkees SA. Putting propylthiouracil in perspective. J Clin Endocrinol Metab.
14. Kowalsky RJ, Falen SW. Radiopharmaceuticals in Nuclear Pharmacy and Nuclear Medicine. 2nd ed. Washington, DC: American Pharmacists Association; 2004.
15. Larose JH. Radionuclide therapy. In: Early PJ, Sodee DB, eds. Principles and Practice of Nuclear Medicine. 2nd ed. St. Louis, MO: Mosby; 1995:752.
16. Culver CM, Dworkin HJ. Radiation safety considerations for post-iodine-131 hyperthyroid therapy. J Nucl Med. 1991;32:169-173.
17. Propranolol hydrochloride package insert. Corona, CA: Watson Laboratories; June 2008.
To comment on this article, contact rdavidson@uspharmacist.com.






