US Pharm. 2010;35(7)(Oncology suppl):4-9.
ABSTRACT: Angiogenesis is essential for tumor development and dissemination. The investigation into the inhibition of tumor angiogenesis has shed new light on cancer treatment. With the approval of 14 molecularly targeted antiangiogenesis therapeutic drugs for cancer treatment, antiangiogenesis therapy represents one of the most significant advances in clinical oncology. Since antiangiogenesis therapy is a targeted therapy aiming at angiogenic microvascular endothelial cells, fewer adverse effects and less drug resistance are associated with antiangiogenesis therapeutic drugs compared to chemotherapy agents. The combination of antiangiogenesis drugs with conventional chemotherapy significantly increases the response rate and quality of life of cancer patients. However, more research is needed to fully understand the biological mechanisms of tumor angiogenesis and optimize this new treatment strategy.
Antiangiogenesis therapies emerged as significant advancements in cancer treatment with the 2004 FDA approval of bevacizumab (Avastin) to treat metastatic colorectal cancer in combination with 5-fluorouracil (5-FU). Bevacizumab is a humanized monoclonal antibody against vascular endothelial growth factor (VEGF). This strategy of stopping tumor growth and metastasis by blocking tumor angiogenesis was pioneered by Judah Folkman, MD, and his colleagues almost four decades ago. A tumor requires and stimulates persistent angiogenesis to supply oxygen and nutrients for its survival and growth and to form a route for tumor cell egress. Antiangiogenesis therapy inhibits tumor growth and restrains metastasis by cutting the fuel supply and destroying the circulating pathway for the tumor cells by blocking tumor angiogenesis.1
Angiogenesis, the growth of new blood vessels from pre-existing vessels, is an important process in both physiologic conditions (i.e., wound healing, reproduction, and embryonic development) and pathophysiologic conditions (i.e., solid tumor growth, psoriasis, and diabetic retinopathy). Angiogenesis is a dynamic and complicated multistep process involving the activation, invasion, migration, proliferation, sprout formation, tube formation, and finally capillary network formation of vascular endothelial cells. All of these steps are essential for the success of angiogenesis and are controlled directly or indirectly by the dynamic balance between angiogenic stimulators and inhibitors. Thus, investigating the cellular and molecular regulatory mechanism of angiogenesis provides potential drug targets to block angiogenesis.2
Sustained tumor angiogenesis is one of the hallmark features of cancer. Tumor angiogenesis refers to the ability of a tumor to stimulate new blood vessel formation to support tumor expansion, local invasion, and dissemination. To grow beyond a critical size or metastasize to another organ, a tumor must stimulate and maintain a network of new blood vessels. The extent of angiogenesis is determined by the balance between the angiogenic stimulators and inhibitors in the microenvironment of the neoplastic tissue. VEGF is one of the key angiogenic stimulators secreted by the tumor cells to switch on the angiogenic phenotype. Intratumor hypoxia and alteration in oncogenes and tumor suppressor genes significantly upregulate VEGF expression. Many cancer cells, including carcinomas of the breast, kidney, colon, brain, ovary, cervix, thyroid, bladder, esophagus, and prostate, overexpress VEGF. In addition, the 3-D extracellular matrix (ECM) environment also plays an active role in the regulation of tumor angiogenesis and tumor invasion. Tumor cells and angiogenic endothelial cells use ECM receptors, such as integrin anb3, to bind the surrounding matrix and invade. They also loosen the surrounding matrix by proteolytically degrading the basement membrane using proteinases such as plasminogen activator, matrix metalloproteinase (MMP), chymase, or heparanase. Furthermore, these proteinases regulate angiogenesis by activating or releasing angiogenic stimulators (i.e., VEGF) sequestered within the ECM. Thus, many investigative antiangiogenesis drugs are targeting angiogenic stimulators, ECM receptors, and ECM proteinases.3,4
Regulation of Angiogenesis
Under normal physiologic conditions, angiogenesis is well controlled by the local balance between endogenous angiogenesis stimulators and angiogenesis inhibitors, although the regulatory mechanism is still not clear. Most of the endogenous angiogenic inhibitors can be categorized as matrix derived, such as endostatin, and nonmatrix derived, such as interferons and angiostatin. At least 30 endogenous angiogenesis inhibitors can be detected in blood circulation, indicating that these angiogenesis inhibitors play an important role in maintaining the angiogenic balance under physiologic conditions. During wound healing, the expression of VEGF, one of the most potent angiogenic stimulators, is significantly upregulated to promote wound healing by restoring blood flow to injured tissues. As wound healing resolves, the expression of VEGF is downregulated and most angiogenic capillaries regress, resulting in a residual normal vascularity.2 New evidence indicates that a number of endogenous angiogenic inhibitors are present in the normal retina to balance the stimulatory effect of VEGF in the regulation of angiogenesis and vascular permeability.5 It suggests that endogenous angiogenic inhibitors can be used to balance the effect of angiogenic stimulators.
Deficient production of angiogenic stimulators turns off the angiogenesis switch and leads to insufficient angiogenesis. Inordinate production of angiogenic stimulators turns on the angiogenesis switch and drives excessive angiogenesis. Both insufficient and excessive angiogenesis contribute to the pathogenesis of many major diseases. Under pathologic conditions, insufficient angiogenesis occurs in coronary artery disease, stroke, and chronic wounds. Excessive angiogenesis occurs mostly when diseased cells, such as inflammatory and tumor cells, produce abnormal amounts of angiogenic stimulators, overwhelming the effects of endogenous angiogenesis inhibitors, thus turning on the angiogenesis switch. Most important, during cancer development the human body loses control over the balance between angiogenesis stimulators and inhibitors, which leads to excessive angiogenesis that feeds the tumor growth and facilitates tumor metastasis. Also of clinical interest, colorectal and breast cancer patients specifically showed increased concentrations in VEGF-A and MMP-9 plasma levels due to malignancy. These levels are reduced after the tumor is removed, which significantly decreases the extent of angiogeneses.6-8
Excessive angiogenesis is seen in diseases such as solid tumor cancers, diabetic retinopathy, age-related macular degeneration, rheumatoid arthritis, and psoriasis, as well as other diseases. In these conditions, new blood vessels feed diseased tissues and destroy normal tissues. In the case of cancer, tumor cells produce and secrete excessive amounts of many potent angiogenic stimulators, such as VEGF, placental growth factor (PlGF), stromal-cell–derived factor 1, and angiopoietin-2. These angiogenic stimulators will activate the complicated multistep angiogenesis process surrounding the tumor microenvironment. New evidence indicates that these angiogenic factors also stimulate the mobilization of cells at distant sites, including bone marrow, into circulation to promote vascularization.9 Moreover, the newly formed angiogenic capillary networks also provide the “superhighway” for the spreading of tumor cells to distant sites through circulation, which is a major cause of high mortality in cancer patients.10
Drug Targets of Antiangiogenesis Therapy
Angiogenesis is a tightly regulated multistep process. Many enzymes, growth factors, ECM proteins, ECM receptors, and their signal transduction pathways involved in the regulation of angiogenesis can be potential targets for angiogenesis therapy. Drugs based on blocking monoclonal antibodies and chemical inhibitors are being developed to counter the effect of angiogenesis growth factors. The principal growth factors driving angiogenesis are VEGF, basic fibroblast growth factor (bFGF), and hepatocyte growth factor/scatter factor. Other positive regulators are angiopoietin-1, angiotropin, angiogenin, epidermal growth factor (EGF), granulocyte colony-stimulating factor, interleukin (IL)-1, IL-6, IL-8, and platelet-derived growth factor (PDGF). Since VEGF plays an essential role in stimulating tumor angiogenesis, blocking VEGF-mediated signaling pathways has been one of the major strategies for antiangiogenesis therapy. Currently, there are six members in the VEGF family (i.e., VEGF-A, -B, -C, -D, -E) and PlGF. These VEGF proteins bind in a distinct pattern to three structurally related receptor tyrosine kinases known as VEGF receptor (VEGFR)-1, -2, and -3. Monoclonal antibodies against VEGF or VEGFR and small molecule inhibitors of VEGFR tyrosine kinase (and its downstream signal transduction pathway) are some of the major antiangiogenesis therapeutic agents.11,12
The first successful treatment of an angiogenesis-dependent disease occurred in 1989, when the drug interferon alfa-2a was used to suppress angiogenesis by inhibiting VEGF and bFGF production, which led to regression of abnormal blood vessels growing in the lungs of a boy with pulmonary hemangiomatosis. In February 2004, bevacizumab (Avastin), a humanized blocking monoclonal antibody for VEGF, was approved to treat metastatic colorectal cancer in combination with 5-FU. Since then, bevacizumab in combination with standard chemotherapy agents has been found efficacious in clinical trials of non–small cell lung cancer (NSCLC), renal cell carcinoma (RCC), glioblastoma, ovarian cancer, and breast cancer. More recently, aflibercept (VEGF Trap) in combination with standard chemotherapy regimens is undergoing phase II and phase III clinical trials in the treatment of advanced solid tumors in five different cancers: colorectal cancer, NSCLC, prostate cancer, pancreatic cancer, and gastric cancer. Aflibercept is a fused protein comprised of segments of the extracellular domains of human VEGFR-1 and VEGFR-2, and constant region (Fc) of human immunoglobulin G (IgG). Aflibercept inhibits angiogenesis by functioning as a soluble decoy receptor to trap VEGFs. Furthermore, the VEGFR inhibitor PTK787/ZK222584 in combination with standard chemotherapy is in phase III clinical trials for colorectal cancer and phase II clinical trials for a number of other tumors, such as metastatic gastrointestinal stromal tumors (GISTs) and unresectable malignant mesothelioma.13-18
In addition to the ligand blocking agents, antiangiogenesis drugs are also being developed to block the signal transduction pathway for angiogenesis stimulators. Several small molecular-weight receptor tyrosine kinase (RTK) inhibitors such as sunitinib (Sutent) and sorafenib (Nexavar), have been developed to target the signal transduction pathway of angiogenic stimulators, such as VEGF, EGF, and PDGF. In 2006, both sunitinib and sorafenib were approved by the FDA for advanced RCC.19,20 In November 2007, the FDA granted approval of sorafenib for the treatment of inoperable hepatocellular carcinoma, and current trials are ongoing in ovarian, pancreatic, and thyroid cancers. In October 2009, the FDA granted approval to pazopanib (Votrient) for the treatment of patients with advanced RCC.21 Pazopanib selectively inhibits VEGFR-1, -2, -3, c-kit (an RTK mutated and constitutively activated in most GISTs), and PDGF receptor, which are all involved in tumor angiogenesis.
A variety of other small-molecule RTK inhibitors targeting the VEGF and EGF receptors signal transduction pathway have been approved by the FDA for the treatment of solid tumor cancers, including gefitinib (Iressa) and erlotinib (Tarceva).22 Nilotinib (Tasigna) inhibits Bcr-Abl kinase, c-kit, and PDGF receptors and is approved by the FDA to treat chronic myelogenous leukemia (CML). Some clinical studies indicate that nilotinib is active in GIST resistant to both imatinib and sunitinib. Other small-molecule agents under clinical investigation include motes-anib, vatalanib, and vandetanib.22,23
The discovery of downstream signal-transduction pathways for RTK has also led to the development of many newly targeted agents. The three major signal transduction pathways for angiogenic growth factors are the Ras/Raf/MAPK pathway, PI3K/Akt/mTOR pathway, and PKC pathway (FIGURE 1). As one of the key protein kinases controlling signal transduction (from various growth factors and upstream proteins to the level of mRNA translation and ribosome biogenesis), mammalian target of rapamycin (mTOR) plays a critical role in regulating cell cycle progression, cellular proliferation and growth, and angiogenesis.24,25 In 2007, the FDA approved temsirolimus (Torisel, also known as CCI-779) as the first drug in the class of mTOR inhibitors for metastatic RCC. Temsirolimus is recommended as first-line treatment for patients with poor-prognosis metastatic RCC. In 2009, the FDA approved everolimus (Afinitor, also known as RAD001) as the second drug in the class of mTOR inhibitors for the treatment of advanced RCC after failure of treatment with sunitinib or sorafenib.24,25
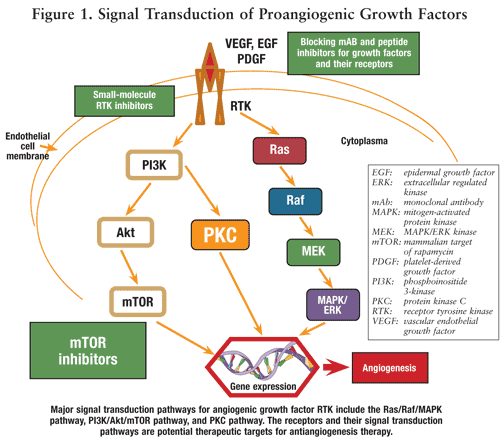
Interestingly, research on some of the previously approved chemotherapeutic agents, such as doxorubicin and cisplatin, demonstrates that they inhibit VEGF production. Thalidomide inhibits angiogenesis mediated by VEGF and bFGF, and studies have shown that thalidomide in combination with dexamethasone has increased the survival of multiple myeloma patients. The combination of thalidomide and dexamethasone is now one of the most common regimens for patients with newly diagnosed multiple myeloma, with a response rate of up to 60% to 70%. In addition to the chemotherapeutic and endogenous angiogenesis inhibitors, natural sources with antiangiogenic properties include tree bark, fungi, shark muscle and cartilage, sea coral, green tea, and herbs such as licorice, ginseng, cumin, and garlic. In total, more than 300 angiogenesis inhibitors have been discovered to date.24-26
Although they may not necessarily directly kill tumor cells, angiogenesis inhibitors significantly enhance the efficacy of standard chemotherapy and radiation therapy by inhibiting tumor growth and tumor metastasis. Therefore, this type of therapy may need to be administered over a long period of time. In the normal healthy body, the process of angiogenesis is dormant and the angiogenesis switch is kept “off” with inhibitors being dominant over stimulators. Since antiangiogenesis therapy is a targeted therapy aimed specifically at the angiogenic stimulators and the angiogenic microvascular endothelial cells, antiangiogenesis therapy usually produces only mild side effects and is less toxic to most healthy cells. But as angiogenesis is important in wound healing and reproduction, long-term treatment with antiangiogenic agents could cause problems with bleeding, blood clotting, heart function, the immune system, and the reproductive system, with some side effects still unknown.27,28
FDA-Approved Antiangiogenesis Agents
Since the time when Dr. Folkman pioneered the concept of antiangiogenesis therapy for cancer treatment four decades ago, angiogenesis research has gained tremendous interest in both academic research institutions and the pharmaceutical industry. Although hundreds of antiangiogenesis therapeutic agents are under investigation, the FDA currently has approved only 14 anticancer drugs with recognized antiangiogenic properties. Based on therapeutic targets, these agents can be grouped into four major categories: monoclonal antibody therapies, small-molecule RTK inhibitors, mTOR inhibitors, and unknown mechanisms.
Monoclonal Antibodies: These agents work by binding biologically active forms of angiogenic stimulators or their receptors and inhibiting endothelial cell proliferation and angiogenesis. Adverse effects of monoclonal antibody therapy are usually fairly mild. Side effects can include fever, chills, weakness, headache, nausea, vomiting, diarrhea, low blood pressure, and rashes (TABLE 1).17
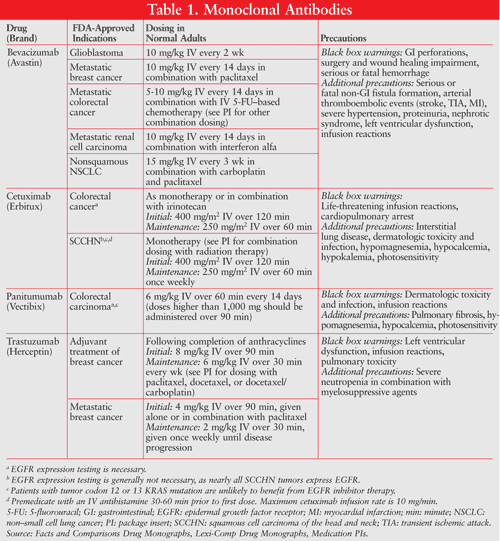
Small-Molecule RTK Inhibitors: This is currently the largest class of drugs that block angiogenesis. These agents have the advantages of hitting multiple targets, oral administration, and potential for lower cost. Lack of target specificity leads to unexpected toxicity but also promising efficacy. Toxicity, notably fatigue, leads to discontinuation in 38% of patients treated with sunitinib. Hypertension, hemorrhage, and cavitation are common toxicities among this class of agents. Rash, fatigue, myalgia, and hand-foot syndrome are more specifically seen with RTK inhibitors. A major adverse effect with the EGF RTK inhibitors is an acnelike rash (TABLE 2).19-23
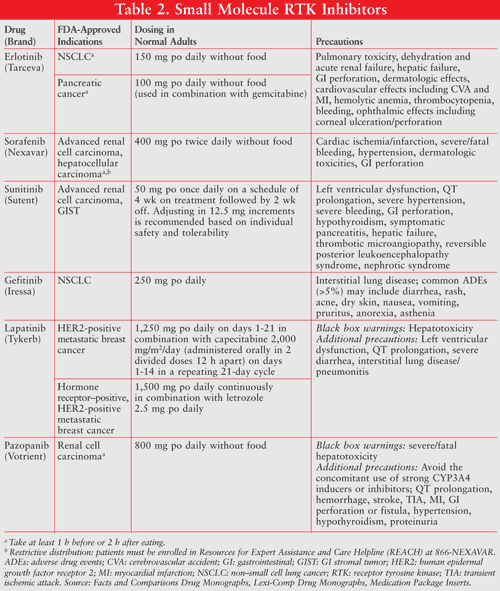
mTOR Inhibitors: These agents represent a third, smaller category of antiangiogenic therapies with two FDA-approved agents, temsirolimus (Torisel) and everolimus (Afinitor). PI3K/Akt/mTOR is one of the three major signaling pathways that have been identified as important in cancer. mTOR is a key kinase downstream of PI3K/Akt, which regulates tumor cell proliferation, growth, survival, and angiogenesis. Since the PI3K/Akt/mTOR pathway is also essential for metabolism, insulin, and insulin growth factor (IGF) signal transduction, hyperglycemia, hypercholesterolemia, and hyperlipidemia are major adverse effects for this drug class. Other common adverse events for temsirolimus and everolimus include fatigue, stomatitis, diarrhea, hypophosphatemia, low red blood cells and platelets, and peripheral edema. These adverse events are commonly reversible upon treatment discontinuation. Less common symptoms are renal insufficiency, interstitial pneumonitis, and low white blood cells (TABLE 3).24-26
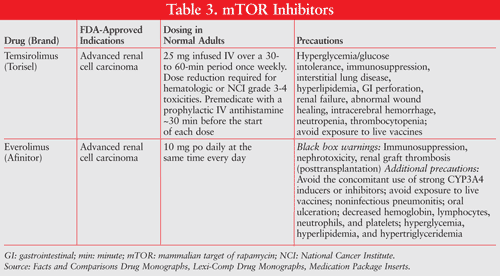
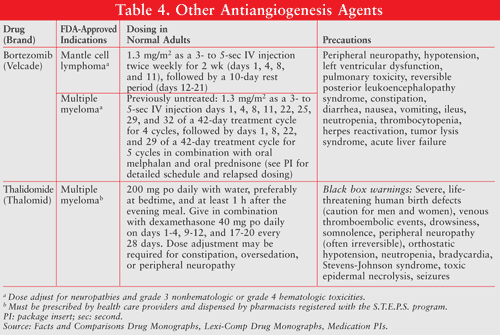
Unknown Mechanisms: Bortezomib (Velcade) and thalidomide (Thalomid) may indirectly inhibit angiogenesis through mechanisms that are not completely understood (TABLE 4).27,28
Summary
Antiangiogenesis therapy represents one of the most significant advances in clinical oncology. It has sparked tremendous interest in angiogenesis research in both academic research institutions and the pharmaceutical industry for the past two decades. The FDA has approved 14 anticancer drugs with recognized antiangiogenic properties. More research is needed to fully understand the biological mechanisms of tumor angiogenesis to optimize this new cancer treatment strategy. Next-generation medications are in development to increase the target specificity and to investigate possible treatments across the spectrum of solid tumors. Although the majority of the currently approved antiangiogenesis drugs only offer a modest survival benefit in a limited patient population, they have paved the way for the development of an optimized antiangiogenesis strategy and improved cancer treatments.
REFERENCES
1. Nussenbaum F, Herman IM. Tumor angiogenesis: insights and innovations. J Oncol.
2. Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Invest Derm. 2000;5:40-46.
3. Tortora G, Melisi D, Ciardiello F. Angiogenesis: a target for cancer therapy. Curr Pharm Des. .
4. Fidler IJ, Lee ME. Neoplastic angiogenesis—not all blood vessels are created equal. N Engl J Med.
5. Ma JX, Zhang SX, Wang JJ. Down-regulation of angiogenic inhibitors: a potential pathogenic mechanism for diabetic complications. Curr Diabetes Rev. 2005;1:183-196.
6. Zaman K, Driscoll R, Hahn D, et al. Monitoring multiple angiogenesis-related molecules in the blood of cancer patients shows a correlation between VEGF-A and MMP-9 levels before treatment and divergent changes after surgical vs. conservative therapy. Int J Cancer. 2006;118:755-764.
7. Pandya NM, Dhalla NS, Santani DD. Angiogenesis—a new target for future therapy. Vascul Pharmacol. 2006;44:265-274.
8. Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947-C970.
9. Semenza GL. A new weapon for attacking tumor blood vessels. N Engl J Med. 2008;358:2066-2067.
10. Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(suppl 16):15-18.
11. Winer E, Gralow J, Diller L, et al. Clinical cancer advances 2008: major research advances in cancer treatment, prevention, and screening—a report from the American Society of Clinical Oncology. J Clin Oncol. 2009;27:812-826.
12. Augustin HG. Translating angiogenesis research into the clinic: the challenges ahead. Br J Radiol.
13. Holden SN, Eckhardt SG, Basser R, et al. Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Ann Oncol.
14. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967-975.
15. Aragon-Ching JB, Dahut WL. VEGF inhibitors and prostate cancer therapy. Curr Mol Pharmacol.
16. Chamberlain MC, Raizer J. Antiangiogenic therapy for high-grade gliomas. CNS Neurol Disord Drug Targets. 2009;8:184-194.
17. Higa G, Abraham J. Biological mechanisms of bevacizumab-associated adverse events. Expert Rev Anticancer Ther. 2009;9:999-1007.
18. Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist. 2007;12:356-361.
19. Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med.
20. Huang D, Ding Y, Li Y, et al. Sunitinib acts primarily on tumor endothelium rather than tumor cells to inhibit the growth of renal cell carcinoma. Cancer Res. 2010;70:1053-1062.
21. Votrient (pazopanib) package insert. Research Triangle Park, NC: GlaxoSmithKline; October 2009. www.accessdata.fda.gov/ 2010;2010:132641 [Epub 2010 Apr 26]. 2004;10:11-26 2004;351:215-216. 2003;76:S3-S10. 2005;16:1391-1397. 2009;2:161-168. 2005;353:172-187.
22. Montemurro M, Schöffski P, Reichardt P, et al. Nilotinib in the treatment of advanced gastrointestinal stromal tumours resistant to both imatinib and sunitinib. Eur J Cancer. 2009;45:2293-2297.
23. Guirgis HM. A perspective on tyrosine kinase inhibitors in gastrointestinal stromal tumors and cancer: past and present with emphasis on future and cost. Cancer Therapy. 2007;5:417-436.
24. Fasolo A, Sessa C. mTOR inhibitors in the treatment of cancer. Expert Opin Investig Drugs. 2008;17:1717-1734.
25. Triano LR, Deshpande H, Gettinger SN. Management of patients with advanced non-small cell lung cancer: current and emerging options. Drugs. 2010;70:167-179.
26. Hudes GR. Targeting mTOR in renal cell carcinoma. Cancer. 2009;115(suppl 10):2313-2320.
27. Board RE, Thatcher N, Lorigan P. Novel therapies for the treatment of small-cell lung cancer: a time for cautious optimism? Drugs. 2006;66:1919-1931.
28. Cabebe E, Wakelee H. Role of anti-angiogenesis agents in treating NSCLC: focus on bevacizumab and VEGFR tyrosine kinase inhibitors. Curr Treat Options Oncol. 2007;8:15-27.
To comment on this article, contact rdavidson@uspharmacist.com.





