US Pharm. 2010;35(9)(Oncology suppl):4-7.
ABSTRACT: Endometrial cancer is the most common invasive gynecologic malignancy in the United States. Although most patients diagnosed with the disease are ≥60 years, the overall 5-year survival rate is high. Risk factors for development include unopposed estrogen, tamoxifen, obesity, high-fat diet, and reproductive factors. Diagnosis is typically made by endometrial histology and biopsy, and treatment depends on the stage of the cancer and grade. Current therapeutic options include surgery, radiation, hormone therapy, and chemotherapy.
Endometrial cancer is the most common invasive gynecologic malignancy in the United States.1,2 In 2010, it is estimated there will be 43,470 new cases and 7,950 deaths associated with the disease.2 This article will review the epidemiology, risk factors, screening, diagnosis, treatment, and future treatment strategies of endometrial carcinoma.
Epidemiology
Endometrial cancer primarily affects postmenopausal women with a mean age of 60 years at diagnosis.2 Although the age-adjusted endometrial cancer incidence in the U.S. has declined since 1975, a transient increase was observed from 1973 to 1978, possibly due to estrogen therapy.2 However, endometrial cancer mortality has decreased from 1974 to the present due to enhanced screening. Most women present with abnormal uterine bleeding, and approximately 75% of patients present with early disease.1 The overall 5-year survival rate for those diagnosed with endometrial carcinoma is 83%.3 Rationale for the high survival rate is early diagnosis.3
Risk Factors
Several pharmacologic agents have been associated with an increased risk in the development of endometrial cancer. Unopposed estrogen therapy in patients with an intact uterus has been associated with an increased risk of endometrial cancer; however, patients taking combination therapy with estrogen and progesterone have also experienced a risk similar to those who do not take postmenopausal therapy.2,4-8 Tamoxifen therapy has also been associated with the development of endometrial cancer. In one study, the risk of endometrial cancer in patients receiving tamoxifen was double compared to those who were receiving placebo (2.30 per 1,000 women compared to 0.91 per 1,000).9
Other factors associated with an increased risk of developing endometrial cancer include obesity, high-fat diet, and reproductive factors (e.g., nulliparity, polycystic ovary syndrome, early menarche, and late menopause).2 Obesity is a major risk factor. Nearly all women with early-stage endometrial cancer are obese.10 In one study, obese women had a higher mortality from medical comorbidities than women who were not obese. Those women with a body mass index (BMI) >40 experienced significantly shorter survival and more endometrial cancer–unrelated deaths in comparison to nonobese women.11 In addition, obesity (e.g., >50 pounds over ideal body weight) can result in endogenous estrogen production because of peripheral conversion of androstenedione to estrone.1
Increased risks of endometrial cancer have also been observed in patients with hereditary nonpolyposis colorectal cancer (HNPCC) syndrome. Patients with this disorder have an estimated cumulative incidence of endometrial cancer from 20% to 60% by age 70 years. The incidence differs slightly based on the germline mutation (for MLH1 carriers the lifetime risk at age 70 years is 25%); however, the MSH2 mutation carriers have a 35% to 40% lifetime risk of endometrial cancer by 70 years.2
Nulliparity and diabetes have been associated with two-to threefold increases in the incidence of endometrial cancer. The incidence of endometrial cancer is lower in African American women; however, African Americans have a less favorable prognosis compared to white women.1,12,13 Survival rates in white women are >10% higher than nonwhite patients at equivalent stages of disease.3
Screening
According to the current American Cancer Society guidelines, screening for endometrial cancer is indicated for women at menopause who are at an average risk.14 These women should be informed about the risks and symptoms of endometrial cancer and are encouraged to report any unexpected bleeding or spotting to their health care provider. Women who are considered high risk for endometrial cancer due to a variety of factors, including known HNPCC genetic mutation carrier status, substantial likelihood of being a mutation carrier (e.g., mutation is present in other family members), or the absence of genetic testing results in families who are suspected to have an autosomal dominant predisposition to colon cancer. High-risk women should begin endometrial cancer screening at age 35 years.14
Diagnosis
Endometrial cancer is diagnosed by endometrial histology with endometrial biopsy.14 Endometrial cancer has been classified into three types. Type I cancers are typically estrogen related and occur in younger, obese, or perimenopausal women. Type I tumors are mostly low grade and arise as background hyperplasia. This is the most common endometrial cancer. Type II disease consists of high-grade tumors, occurs in older patients, and is more common in women of color. Type II disease occurs in up to 10% of cases.1 The third type of endometrial cancer is hereditary or genetic and can have a familial association or be a part of HNPCC, also known as Lynch II syndrome. Genetic disease represents up to 10% of cases, of which 5% are Lynch II syndrome.1 Characteristics of type 1 and type II endometrial cancers are listed in TABLE 1.1,15
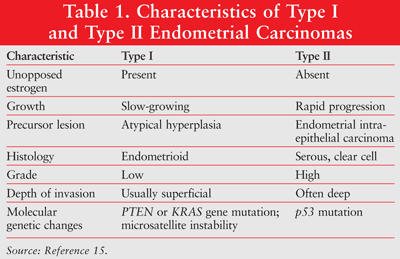
Diagnosis is usually made by biopsy. The procedure involves the acquisition of peritoneal fluid or washings and abdominal cavity, pelvic, and para-aortic nodal exploration. A total hysterectomy is also typically conducted if a malignancy is found.16
Endometrial cancer is graded from least to most aggressive according to histologic differentiation. This is evidenced by grade 1 (G1; nonsquamous, nonmorular solid growth pattern comprises £5% of the tumor); grade 2 (G2; nonsquamous, nonmorular solid growth pattern comprises 5%-50% of the tumor); and grade 3 (G3; nonsquamous, nonmorular solid growth pattern comprises >50% of the tumor). Endometrial cancer is also designated by low, intermediate, or high risk (TABLE 2).16,17
After endometrial cancer has been eradicated, women should repeat examinations every 3 to 4 months for the first 2 years, every 6 months for the next 3 years, and annually after 5 years. In addition, vaginal cytology should be performed every year.1
Treatment
Depending on the stage of the cancer and histologic grade, surgery, radiation therapy, hormone therapy and chemotherapy are used as monotherapy or sequentially.3 In early disease, surgery is the primary treatment; however, certain exceptions to surgery exist. Patients at increased risk for mortality or severe morbidity secondary to comorbidities may elect other treatment options (e.g., radiation therapy, chemotherapy).1 Appropriate therapeutic options for patients with endometrial cancer based on stage of clinical disease are described in TABLE 2.16,17
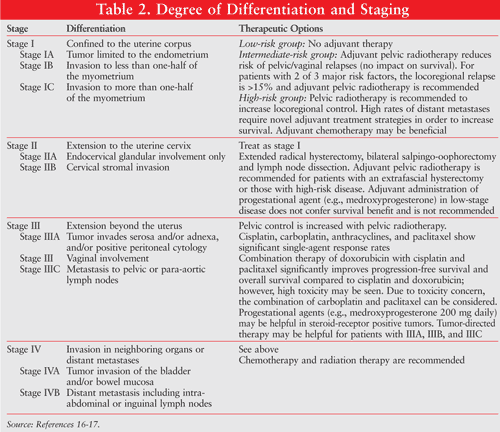
The use of radiation therapy for patients with stage IB, IC, and III endometrial cancers is controversial. External radiation therapy has been used successfully to prevent local recurrences; however, overall improvements in survival have not been observed.18-20 Most of the controversy regarding the use of external radiation therapy centers on which patient populations to include (high-risk uterine factors, node positive, cervix involvement), which fields (pelvic, vaginal cuff, combinations), and the timing related to chemotherapy (before, during, or after).21
Other research indicates that stage IC or G3 endometrial cancers are at high risk of early distant spread and endometrial-cancer related death, and novel strategies for adjuvant therapy should be explored in this patient population. Others continue to recommend radiation therapy for stage IC or G3 endometrial cancers, especially since adjuvant radiation has little impact on overall survival.22
Several studies have evaluated the role of adjuvant radiation in early and advanced endometrial cancer. In one study, external radiation reduced vaginal and pelvic disease recurrence in patients with stage I disease; however, distant metastases and 5-year survival were not improved.23 Similar results were obtained in another study with stage IB (G2 or G3)/stage IC (G1 or G2) endometrial cancer. Although pelvic radiation improved local disease, no improvement on distant metastases or survival rates was observed.24 Keys et al found a beneficial effect on recurrence-free survival compared to no adjuvant treatment (HR = 0.42; 92% CI, 0.25-0.73; P = .007), but therapy did not significantly improve 5-year survival rates (92% vs. 86%; P = .557).18
Multiple-agent chemotherapy regimens are preferred, if tolerated.17 The following designations are used by the National Comprehensive Cancer Network (NCCN) for the categories of evidence and consensus. Category 1 means that the recommendation is based on a high level of evidence (e.g., randomized controlled trials) with uniform NCCN consensus. Category 2A is based on lower-level evidence with uniform NCCN consensus. Category 2B means that the recommendation is based on lower-level evidence with nonuniform NCCN consensus (but no major disagreement). Category 3 is based on any level of evidence but reflects major disagreement. (All recommendations are designated as category 2A unless otherwise noted.) Combination therapy with cisplatin/doxorubicin (category 1), cisplatin/doxorubicin/
Single-agent therapies, including cisplatin, carboplatin, doxorubicin, epirubicin, fluorouracil, paclitaxel, and docetaxel, have a response rate >20%.17,22 However, the optimal chemotherapeutic regimen is controversial due to significant toxicity associated with these agents and comorbid disease states of patients with endometrial cancer.22
Doxorubicin-cisplatin combination therapy results in a significantly higher response rate than doxorubicin monotherapy.25,26 The response rate and overall success by 24-hour paclitaxel-doxorubicin and filgrastim were equivalent to that produced by the combination of doxorubicin-cisplatin.27 In addition, the concomitant use of paclitaxel with doxorubicin-cisplatin therapy has been shown to improve the objective response and overall survival in women with advanced or recurrent endometrial carcinoma.28 Refer to TABLE 3 for common chemotherapy dosing strategies.
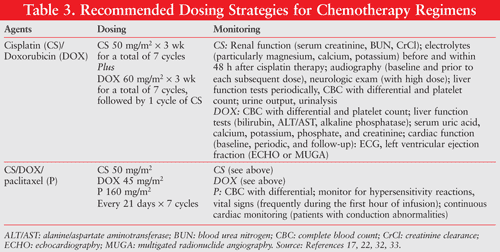
Hormone therapy is typically reserved for patients with advanced or recurrent disease and those with low-grade, hormone receptor–positive endometrioid adenocarcinomas.17,29 The median duration of response generally does not exceed 10 months.15 Typically a progestational agent is used first-line, followed by tamoxifen therapy; however, results with tamoxifen were inferior to those obtained with progesterones.15,30 Although aromatase inhibitors have been used, data supporting their use are limited and response rates have been low (9.4%).15,30
Future Therapeutic Options
Several agents are currently being considered for the treatment of endometrial carcinoma, both alone and in combination therapy with cytotoxic agents.31 Among the agents that have been studied are trastuzumab, faslodex, thalidomide, gefitinib, lapatinib, bevacizumab, VEGF-Trap, ixabepilone, and gemcitabine. Although other biologic therapy has been evaluated, biologic and molecular therapies do not have sufficient evidence in the treatment of recurrent or metastatic endometrial carcinoma.17 Current evidence suggests that these agents may be most effective when given in combination with existing cytotoxic agents or another novel therapeutic agent. In the future, patients may benefit from the application of innovative combinations of pharmaceutical agents (concurrently or in sequence) and the development of new molecular and genetic targets for endometrial cancer.21
Conclusion
Endometrial cancer is common among American women. The overall 5-year survival rate of this disease is 83% due to early detection.3 Several pharmacologic agents and lifestyle risk factors may predispose patients to endometrial cancer. Current treatment options include surgery, radiation, chemotherapy, and hormonal therapy. Novel therapies for the treatment of endometrial cancer are needed and will focus on the sequence in which agents are delivered and identifying molecular and genetic markers to target the cancer.
REFERENCES
1. Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436-447.
2. Endometrial cancer screening. Epidemiology of endometrial cancer. National Cancer Institute. www.cancer.gov/cancertopics/
3. Dizon DS. Treatment options for advanced endometrial carcinoma. Gynecol Oncol. 2010;117:373-381.
4. Pike MC, Peters R, Cozen W, et al. Estrogen-progestin replacement therapy and endometrial cancer. J Natl Cancer Inst. 1997;89:1110-1116.
5. Persson I, Weiderpass E, Bergkvist L, et al. Risks of breast and endometrial cancer after estrogen and estrogen-progestin replacement. Cancer Causes Control. 1999;10:253-260.
6. Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036-1045.
7. Doherty JA, Cushing-Haugen KL, Saltzman B, et al. Long-term use of postmenopausal estrogen and progestin hormone therapies and the risk of endometrial cancer. Am J Obstet Gynecol. 2007;197:139.e1-e7.
8. Barakat RR, Bundy BN, Spirtos NM, et al. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer. J Clin Oncol. 2006;24:587-592.
9. Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer—96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272-282.
10. Courneya KS, Karvinen K, Campbell K, et al. Associations among exercise, body weight, and quality of life in a population-based sample of endometrial cancer survivors. Gynecol Oncol. 2005;97:422-430.
11. Von Gruenigen VE, Tian C, Frasure H, et al. Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma. Cancer. 2006;107:2786-2791.
12. Partridge EE, Shingleton H, Menck H. The National Cancer Data Base report on endometrial cancer. J Surg Oncol. 1996;61:111-123.
13. Hill H, Coates R, Austin H, et al. Racial differences in tumor grade among women with endometrial cancer. Gynecol Oncol. 1995;56:154-163.
14. Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2009;59:27-41.
15. Chu CS, Lin L, Rubin SC. Cancer of the uterine body. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 8th ed. Philadelphia, PA: Wolters Kluwer; 2008:1545.
16. Baekelandt M, Castiglione M; ESMO Guidelines Working Group. Endometrial carcinoma: ESMO clinical recommendations for diagnosis, treatment, and follow-up. Ann Oncol. 2009;19(suppl 2):119-120.
17. NCCN Clinical Practice Guidelines in Oncology. Uterine neoplasms. V.1.2010. www.nccn.org. Accessed May 5, 2010.
18. Keys HM, Roberts JA, Brunetto V. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma. Gynecol Oncol. 2004;92:744-751.
19. Creasman W, Morrow C, Bundy B, et al. Surgical pathologic spread patterns of endometrial cancer. Cancer. 1987;60(suppl 8):2035-2041.
20. Creutzberg CL, van Putten WL, Koper PC. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicenter randomized trial. PROTEC Study Group. Lancet. 2000;35:1404-1411.
21. McMeekin DS. Where is the future of endometrial cancer therapy? Ann Oncol. 2009;20:1757-1791.
22. Kodama J, Seki N, Hiramatsu Y. Chemotherapy for high-risk early-stage endometrial cancer. Curr Opin Obstet Gynecol. 2007;19:42-47.
23. Aalders J, Abeler V, Kolstad P, Onsrud M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980;56:419-427.
24. Creutzberg CL, van Putten WL, Warlam-Rodenhuis CC, et al. Outcome of high-risk stage IC, grade 3, compared with stage I endometrial carcinoma patients: the Postoperative Radiation Therapy in Endometrial Carcinoma Trial. J Clin Oncol. 2004;22:1234-1241.
25. Thigpen JT, Brady MF, Homesley HD. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma. J Clin Oncol. 2004;22:3902-3908.
26. Aapro MS, van Wijk FH, Bolis G. Doxorubicin versus doxorubicin and cisplatin in endometrial carcinoma: definitive results of a randomised study (55872) by the EORTC Gynaecological Cancer Group. Ann Oncol. 2003;14:441-448.
27. Fleming GF, Filiaci VL, Bentley RC. Phase III randomized trial of doxorubicin + cisplatin versus doxorubicin + 24 hour paclitaxel + filgrastim in endometrial carcinoma. Ann Oncol. 2004;15:1173-1178.
28. Fleming GF, Brunetto VL, Cella D. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma. J Clin Oncol. 2004;22:2159-2166.
29. Van Wijk FH, van der Burg ME, Burger CW, et al. Management of recurrent endometrial carcinoma: an overview. Int J Gynecol Cancer. 2009;19:314-320.
30. Ma BB, Oza A, Eisenhauer E, et al. The activity of letrozole in patients with advanced or recurrent endometrial cancer and correlation with biological markers—a study of the National Cancer Institute of Canada Trials Group. Int J Gynecol Cancer. 2004;14:650-658.
31. Gehrig PA, Bae-Jump VL. Promising novel therapies for the treatment of endometrial cancer. Gynecol Oncol. 2010;116:187-194.
32. Humber CE, Tierney JF, Symonds RP, et al. Chemotherapy for advanced, recurrent or metastatic endometrial cancer: a systematic review of Cochrane collaboration. Ann Oncol. 2007;18:409-420.
33. Lexi-Comp Online [subscription required]. www.crlonline.com.ezproxy.
To comment on this article, contact rdavidson@uspharmacist.com.





