US Pharm. 2011;36(3)(Oncology suppl):3-8.
ABSTRACT: Pancreatic cancer usually presents with no symptoms or vague, nonspecific symptoms, which makes early diagnosis very difficult. Because of this, it produces a high mortality rate. Even with a localized, resectable tumor, median survival is only 24 months. The causes are not well understood, but they include smoking and heredity. Surgery and chemotherapy are the mainstays of treatment, and pharmacists can assist patients and caregivers through drug therapy monitoring and counseling.
Risk Factors
Pancreatic cancer is a complex disease. Risk factors include environmental and genetic factors that are not well understood. Tobacco use is strongly associated with an increased risk of pancreatic cancer. The risk appears to be at least 2.5 times greater than that of a nonsmoker, with a direct correlation between amounts and duration of tobacco use.4 Dietary relationships such as high-fat, high-cholesterol foods (meat and dairy) and the role of alcohol, caffeine, and aspirin have all been inconclusive.5
It is arguable whether there are any diseases involved in the development of pancreatic cancer. Some studies suggest that patients with a history of chronic pancreatitis, chronic cirrhosis, and diabetes are at an increased risk.5 Others argue that these studies have too many confounding factors, and better-controlled studies need to be conducted before making any conclusion. Interestingly, a recent study suggests that patients with type A, B, or AB blood are at an increased risk of developing pancreatic cancer compared to those with type O blood.6
Etiology of Pancreatic Cancer
Pancreatic cancer can arise from various tissues within the secretory gland (pancreas). The type of cancer can be divided into those with predominantly exocrine or endocrine tissue differentiation, with the exocrine being further divided into solid and cystic tumors. Greater than 90% of pancreatic cancers are ductal adenocarcinoma, which is the most common solid, exocrine tumor of the pancreas.8 Other types of exocrine pancreatic cancer include acinar cell and pancreatoblastoma. This article will focus on ductal adenocarcinoma.
Symptoms
The symptoms of pancreatic cancer depend on the stage and location of the tumor. Many patients present with vague and nonspecific symptoms that make pancreatic cancer difficult to diagnose. Weight loss, abdominal discomfort, and nausea are common symptoms, and these often occur late in the disease.10 Since pancreatic cancer often involves the head of the pancreas, jaundice secondary to obstructive cholestasis may present. Duodenal obstruction can precipitate nausea and vomiting. Patients often describe the pain that accompanies pancreatic cancer as a deep, dull, constant, upper abdominal pain that radiates to the middle of the upper back. Patients who present with a sudden onset of type 2 diabetes should be thoroughly assessed for pancreatic cancer, as there may causative link.11 Unfortunately, there are no early warning signs and symptoms of pancreatic cancer that have been identified.
Screening
There is a need for an effective screening tool for pancreatic cancer. Most patients are diagnosed with advanced disease in which 5-year survival is less than 5%.2 Screening studies are trying to identify patients with early- disease pancreatic cancer, with the hopes of improving survival outcomes. Currently, there is no universal primary screening test recommended for pancreatic cancer. Studies are investigating the role of endoscopic ultrasonography (EUS), MRI, and biomarkers in predicting early pancreatic cancer. Some institutions are focusing on surveillance of high-risk individuals. The use of EUS in high-risk asymptomatic patients (first-degree relative, chronic pancreatitis, etc.) may be helpful in detecting early pancreatic cancer.10 The tumor marker, cancer antigen-19-9 (CA-19-9), is often elevated in pancreatic cancer and other malignancies. Because of its low specificity, it is not useful as a screening biomarker for pancreatic cancer. Studies suggest that CA-19-9 does have applications when evaluating therapeutic response to treatment of pancreatic cancer.11
Diagnosing and Staging
Any patient with signs and/or symptoms suggestive of pancreatic cancer should undergo a more thorough workup. A patient with suspected pancreatic cancer should be evaluated with a triphasic, multidetector helical CT scan using contrast material.11 A CT scan is helpful in diagnosing and staging pancreatic cancer based on resectability of the tumor. If a patient cannot tolerate contrast media, then an MRI or EUS may be considered. Endoscopic retrograde cholangiopancreatography may be helpful in placing an endoscopic stent to relieve obstruction.5
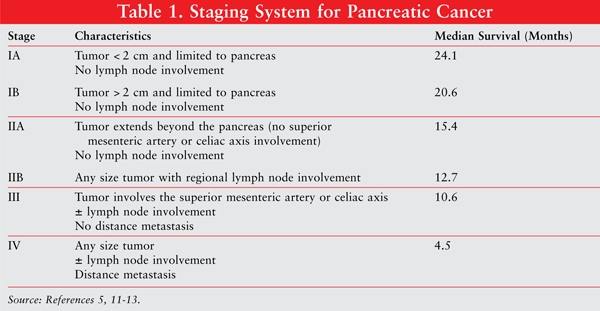
Pancreatic cancer is staged according to the Tumor Node Metastasis staging system from the American Joint Committee on Cancer (AJCC). The most clinically important distinction is whether or not the tumor is resectable. A stage III tumor is classified as unresectable locally advanced disease, and patients with stage IV pancreatic cancer have distance metastasis.12 See TABLE 1 for a more thorough description.
Prognosis
Because pancreatic cancer is generally asymptomatic until a late-stage diagnosis is made, the prognosis for most patients is quite poor. Fewer than 20% of all patients with pancreatic cancer are diagnosed with early, localized disease that can be potentially cured.2 Most patients are diagnosed with advanced disease where survival is less than 1 year. An even dimmer picture is for the 53% of patients diagnosed with stage IV pancreatic cancer, where median survival is 4.5 months.2,13 Additional median survival data for the specific stages can be found in TABLE 1.
Treatment
Surgery and chemotherapy are the main treatment modalities for patients with pancreatic cancer. Surgery is the treatment of choice for patients with resectable disease since it increases the likelihood of a cure.11 There are many surgical procedures used for pancreatic cancer resection depending on the location of the tumor. A pancreaticoduodenectomy (Whipple procedure) is one of the most common procedures, in which the head of the pancreas, duodenum, proximal jejunum, gallbladder, common bile duct, and distal stomach are removed. A distal pancreatectomy is performed when the tumor is found in the tail of the pancreas.14 Mortality rates can be as high as 16% in patients undergoing a pancreaticoduodenectomy for early-stage cancer. Studies suggest an inverse correlation between the number of Whipple procedures performed at a given institution and the mortality rate. Therefore, careful selection of a surgeon and institution should be considered.15 As with many cancers, local recurrence occurs in most patients with pancreatic cancer even after surgery. Postoperative systemic chemotherapy should be considered for these patients.11,14 TABLE 2 provides an overview of the chemotherapeutic agents FDA approved for pancreatic cancer.
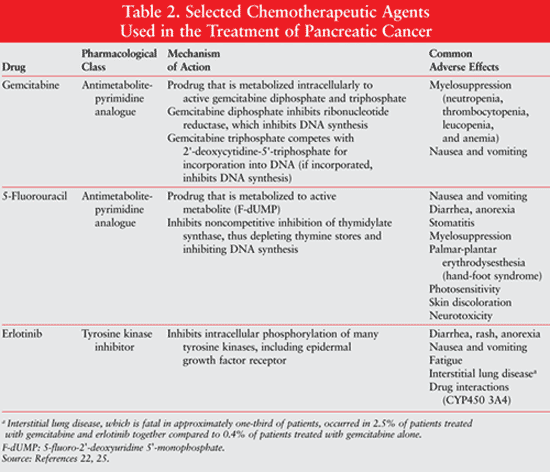
Early-Stage Pancreatic Cancer: Stage I and II pancreatic cancers are considered early-stage pancreatic cancers and are associated with resectable disease. As mentioned above, surgery is the treatment of choice for curative intent. Even with surgery, the 5-year survival rate is no more than 30%. Therefore, additional treatment measures are needed.2,11 Several studies have examined the role of postoperative adjuvant chemotherapy in patients with resected pancreatic cancer. These findings have been conflicting, creating controversy about the management of early disease. Three large phase III trials have examined the role of chemotherapy and chemoradiation in the treatment of postoperative pancreatic cancer. The European Study Group for Pancreatic Cancer 1 trial (ESPAC-1) found that postoperative chemotherapy (5-fluorouracil + leucovorin or gemcitabine) improved progression-free and overall survival in patients who had undergone resection. After a median follow-up of 47 months, the median survival was 20.1 months in patients who received chemotherapy compared to 15.5 months without chemotherapy. The 5-year survival was 21% in the chemotherapy group versus 8% in the group without chemotherapy. Interestingly, the combination of radiation and chemotherapy worsened survival.16 The CONKO-001 trial was a multicenter trial that compared six cycles of postoperative gemcitabine compared to observation alone. There was a difference between median disease-free survival between the groups (13.4 months with gemcitabine compared to 6.9 months with observation alone; P <.001). However, there was no difference in overall survival (median of 22.1 compared to 20.2 months, respectively).17 The Radiation Therapy Oncology Group trial, RTOG-9704, assigned postoperative patients to either adjuvant 5-fluorouracil (5-FU) or adjuvant gemcitabine. Both groups received concurrent chemoradiation (radiation and 5-FU). The median survival was 20.5 months for gemcitabine and 16.9 months for 5-FU (P = 0.09).18 Although these trials do not clarify the role of postoperative chemotherapy, they do suggest a trend that chemotherapy may improve outcomes. The National Comprehensive Cancer Network (NCCN) guidelines suggest that adjuvant chemoradiation with 5-FU or chemotherapy alone with gemcitabine is an option for patients with resectable pancreatic cancer.11
Locally Advanced and Metastatic Disease: Because of the vague and nonspecific symptomatology of pancreatic cancer, the vast majority of patients will be diagnosed with unresectable, locally advanced, or metastatic disease. The treatment of locally advanced disease is similar to that used for metastatic disease since it is difficult to enroll the low number of patients with locally advanced disease in clinical trials. Most patients will present with pancreatic cancer that has metastasized. Two common sites of metastases are the liver and peritoneal cavity. Systemic chemotherapy or chemoradiation is used in the management of the disease.11 The goals of chemotherapy are for palliation and have less of an impact on survival. Gemcitabine and 5-FU are the foundation for most regimens, with gemcitabine preferred when used alone. In a phase III trial, gemcitabine was compared to bolus 5-FU in patients with advanced pancreatic cancer. The primary end point was defined as a clinical benefit response (pain intensity, analgesic use, and quality of life performance). Gemcitabine was found to be significantly superior to 5-FU, with a clinical response benefit of 23.8% with gemcitabine compared to 5% with 5-FU. Median survival was improved with gemcitabine compared to 5-FU (5.7 months compared to 4.4 months, respectively).19 Due to the results of this trial, gemcitabine became the treatment of choice for most patients with advanced pancreatic cancer.
Recent studies suggest the rate of infusion of gemcitabine may affect outcomes. By infusing gemcitabine at a fixed-dose rate (FDR) of 10 mg/m2/min, the intracellular levels of active (phosphorylated) gemcitabine are maximized. It is thought that higher intracellular concentrations of active gemcitabine may correlate with antitumor activity. A phase II trial showed that gemcitabine given over an FDR was associated with a higher response rate and better survival than gemcitabine when given over 30 minutes.11 A subsequent phase III trial supported these findings, with FDR gemcitabine increasing survival over standard dose gemcitabine in advanced pancreatic cancer (6.2 months vs. 4.9 months, P = .04 not statistically significant since P <.025 was required per protocol).20
Several studies have examined gemcitabine in combination with other chemotherapeutic or biologic agents. Numerous studies have investigated gemcitabine in combination with a fluoropyrimidine such as 5-FU. Although not statistically significant, there appears to be a trend toward improved survival when capecitabine or 5-FU is added to gemcitabine. NCCN guidelines suggest that this combination may be an option for those patients wishing aggressive therapy outside of a clinical trial.11 Other agents have also been utilized. Several phase III trials failed to show survival benefit when combining cisplatin and gemcitabine.11 A phase III trial of gemcitabine compared to gemcitabine in combination with oxaliplatin in patients with advanced pancreatic cancer showed that the combination of gemcitabine and oxaliplatin was superior in the endpoints of response rate, progression-free survival, and clinical benefit. Overall survival was not significant between the groups (9.0 months in the combination group, 7.1 months with gemcitabine alone; P = .13).21 NCCN recommends that a platinum-based agent be considered in patients who have pancreatic cancer with hereditary risk factors that suggest platinum-sensitive disease (such as BRCA mutations).11
A phase III study of gemcitabine compared to the combination of gemcitabine and erlotinib showed an improvement in overall survival (median survival 5.91 months vs. 6.37 months). One-year survival rates were also significant (17% vs. 24%, respectively).22 This led to the FDA approval of the combination of erlotinib and gemcitabine for locally advanced, unresectable, or metastatic pancreatic cancer. The use of the biologics bevacizumab and cetuximab have not shown benefit. An abstract at the American Society of Clinical Oncologists meeting in 2010 reported results of the phase III trial, PRODIGE 4/ACCORD 11. This trial randomized 342 patients with metastatic pancreatic cancer to either gemcitabine or FOLFIRINOX (5-FU, leucovorin, irinotecan, and oxaliplatin). An interim analysis recommended halting the study because data indicated that patients receiving FOLFIRINOX had an improved response rate, improved disease control, and longer median progression-free survival. Overall survival was extended from 6.8 months in the gemcitabine arm to 11.1 months with FOLFIRINOX (P <.0001).23 This is the first phase III trial to ever show an 11-month survival benefit in metastatic pancreatic cancer (stage IV). It is important to note that patients receiving FOLFIRINOX had higher rates of grade 3 or 4 febrile neutropenia, which can be fatal. Therefore, proper patient selection is critical.
Clinical Studies
There are over 800 current clinical trials investigating drug therapy and pancreatic cancer.24 Unlike many cancers where clinical trials are often reserved for patients in whom initial therapy has failed, clinical trials should be discussed up front with those who have pancreatic cancer. Since pancreatic cancer is associated with poor outcomes, NCCN guidelines recommend that patients with good performance status consider enrolling in a clinical trial.11 These trials continue to investigate the role of biologic agents, improved chemotherapy combinations, and the use of vaccines.
Pharmacist Involvement
Pharmacists can play an integral role in the treatment of pancreatic cancer. In addition to the chemotherapy agents and side effects that accompany these agents, pharmacists can address the supportive care needs of the patient, many of which necessitate the use of medication. Drug therapy monitoring can be a very important function for dealing with pancreatic cancer. TABLE 3 summarizes the supportive care needs of pancreatic cancer patients.
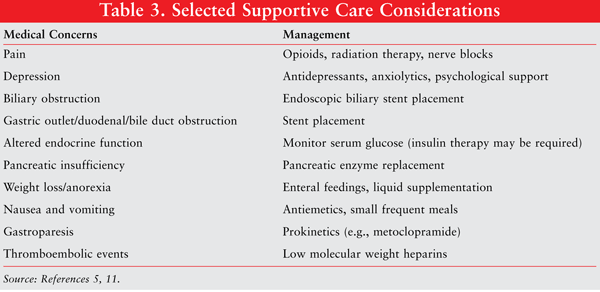
The pharmacist must consider the treatment modalities utilized, including surgery, radiation, and chemotherapy. For instance, patients who have surgery are at risk for pancreatic insufficiency and may require pancreatic enzyme replacement to be taken with food to help with the absorption of fats and proteins. Pain control is one of the most significant issues reported in patients with pancreatic cancer. Pharmacists can be involved in the management of pain utilizing opioids to control pain as well as addressing subsequent adverse effects from opioid therapy. Patients with pancreatic cancer are also at a high risk of thromboembolic events. NCCN guidelines recommend low molecular weight heparin as the preferred treatment when patients experience a thromboembolic event.11 Pharmacists can be always be available to provide informative about the chemotherapeutic, analgesic, and other adjuvant agents prescribed.
REFERENCES
1. Jemal A, Siegal R, Ward E, et al. Cancer statistics 2010. CA Cancer J Clin. 2010;60:277-300.
2. Altekruse SF, Kosary CL, Krapcho M, et al. SEER cancer statistics review, 1975-2007, National Cancer Institute, Bethesda, MD. http://seer.cancer.gov/csr/
3. Arnold LD, Patel AV, Jacobs EJ, et al. Are racial disparities in pancreatic cancer explained by smoking and overweight/obesity? Cancer Epidemiol Biomarkers Prev. 2009;18(9):2397-2405.
4. Hassan MM, Bondy ML, Wolff RA, et al. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol. 2007;102:2696-2707.
5. Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617.
6. Wolpin BM, Chan AT, Hartge P, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101:424-431.
7. Chanjuan S, Hruban RH, Kline AP. Familial pancreatic cancer. Arch Pathol Lab Med. 2009;133:365-374.
8. Yeo CJ, Pluth-Yeo T, Hruban RH, et al. Cancer of the pancreas. In: Devita VT, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 7th ed. Philadelphia, PA: Lippincott; 2005:945-986.
9. Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133:413-422.
10. Irisawa A, Sato A, Sato M, et al. Early diagnosis of small pancreatic cancer: role of endoscopic ultrasonography. Digestive Endoscopy. 2009;21(suppl 1):S92-S96.
11. National Comprehensive Cancer Network Practice Guidelines. Pancreatic cancer. Rockland, PA. Version 2, 2010. www.nccn.org/professionals/ physician_gls/f_guidelines.asp Accessed December 20, 2010.
12. Exocrine and endocrine pancreas. In: Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:241-249.
13. Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC pancreatic cancer staging system: report from the National Cancer Database. Cancer. 2008;110:738-744.
14. Yokoyama Y, Nimura Y, Nagino M. Advances in the treatment of pancreatic cancer: limitations of surgery and evaluation of new therapeutic strategies. Surg Today. 2009;39:466-475.
15. Birkmeyer JD, Finlayson SR, Tosteson AN, et al. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery. 1999;125(3):250-256.
16. Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200-1210.
17. Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267-277.
18. Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299(9):1019-1026.
19. Burris HA III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403-2413.
20. Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:3778-3785.
21. Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509-3516.
22. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared to gemcitabine alone in patients with advanced pancreatic cancer. A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966.
23. Conroy T, Desseigne F, Ychou M, et al. Randomized phase III trial comparing FOLFIRINOX (F: 5FU/leucovorin [LV], irinotecan [I], and oxaliplatin [O]) versus gemcitabine10 as first-line treatment for metastatic pancreatic adenocarcinoma (MPA): Preplanned interim analysis results of the PRODIGE 4/ACCORD 11 trial. J Clin Oncol. 2010;28:15s (suppl; abstract 4010).
24. Clinical trials Web site: www.clinicaltrials.gov. Accessed December 20, 2010.
25. Micromedex Healthcare Series [intranet database]. Greenwood Village, CO: Thomson Healthcare. Accessed December 20, 2010.
To comment on this article, contact rdavidson@uspharmacist.com.





