US Pharm. 2011;36(5)(Oncology suppl):12-15.
ABSTRACT: Chemotherapy-induced nausea and vomiting (CINV) is a major side effect of cancer therapy. Research is under way to develop ideal antiemetics, and recently the FDA has approved drugs that make chemotherapy more tolerable. This review looks at some of the older drugs as well as more recent agents on the market. The 5-hydroxy-tryptamine-3 (5-HT3) receptor antagonists are landmark drugs in this field and are discussed in depth along with the newer neurokinin-1 (NK1) receptor antagonists.
Nausea and vomiting (NV) have been identified by patients as the most distressing side effect of cancer chemotherapy.1 Chemotherapy-induced nausea and vomiting (CINV) is experienced by 70% to 80% of chemotherapy patients. This figure is influenced by the repeated chemotherapy cycles, patient-specific risk factors, and the particular agent. For example, cisplatin is considered the most emetic chemotherapy agent and is therefore the most commonly used agent for the clinical testing of antiemetic agents.2
Approximately 46% of patients have been known to discontinue therapy due to the severity of CINV, and about 50% may completely avoid chemotherapy for the same reasons.1 Pharmacists therefore have an extensive role to play in improving the adherence to chemotherapy through effective counseling.
Poorly controlled emesis results in a diminished quality of life and increased health care costs.3 In the United States, over $1.5 billion is currently spent on CINV management.4 Apart from the stress and anxiety related to it, NV can cause dehydration and malnourishment.5 Thus, it is critical that CINV be optimally managed.
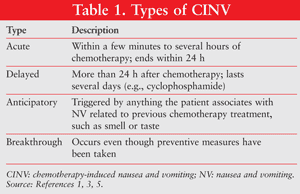
Types of CINV
The NV can be described as acute, delayed, anticipatory, or breakthrough, with delayed and anticipatory being the most frequently encountered in ambulatory care.1,3,5 These are briefly described in TABLE 1.
More often than not, the emesis is cyclical. For example, cisplatin causes acute NV on day 1 followed by delayed NV on day 3.2 It is important to take this into consideration when recommending appropriate antiemetic agents.
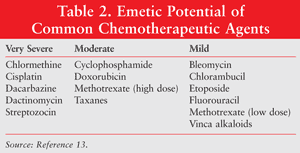
Chemotherapeutic agents cause varying degrees of emesis. The emetic potential of some commonly used chemotherapeutic agents is listed in TABLE 2.
Mechanism of CINV
While the exact mechanism of CINV is not known, it is thought to occur through several noxious actions and neuronal pathways.6 The chemoreceptor trigger zone, located outside the blood–brain barrier, senses emetic stimuli.7 It is home to a number of receptors that are involved in emesis, including serotonin-3 (5-hydroxytryptamine-3 [5-HT3]), neurokinin-1 (NK1), dopamine-2 (D2), and muscarinic-cholinergic receptors. Cytotoxins stimulate the release of chemicals such as serotonin that bind to these receptors and initiate a response.7 Additionally, there is a peripheral pathway that works through the activation of neurotransmitters by local gastrointestinal (GI) irritation or damage. Since a number of neurotransmitters are involved in these processes, an ideal agent would modulate all these systems.
Risk Factors for CINV
The risk for CINV is dependent upon the emetic potential of the chemotherapy regimen as well as patient-specific factors. Young patients, female patients, and those who have experienced CINV during previous therapy are at the highest risk.7 Furthermore, those patients who have a previous history of motion sickness or have experienced emesis during pregnancy are at a higher risk of developing CINV.2
Treatment of CINV
Ideally, an antiemetic should:
• Provide complete control over NV
• Be free of any side effects
• Be easy to use
• Be affordable.
There are a number of drug classes with antiemetic activity, but none of the drugs currently available has all of the above listed qualities.2 Drugs that have been used at some stage or another for CINV control include phenothiazines, buty-rophenones, substituted benzamides, antihistamines, corticosteroids, anticholinergics, cannabinoids, 5-HT3 receptor antagonists, benzodiazepines, and NK1 receptor antagonists.8
Phenothiazines, such as prochlorperazine, were the first group of drugs to be used in the management of CINV.9 In the late 1970s and early 1980s, metoclopramide was used alone or in combination with a corticosteroid such as dexamethasone as the antiemetic choice for CINV management.10 In the late 1980s and early 1990s, the 5-HT3 receptor antagonists were shown to be even better at managing emesis and were associated with the fewest side effects.10 Furthermore, it was demonstrated that the addition of a D2 receptor antagonist or dexamethasone improves the efficacy of 5-HT3 receptor antagonists.9 It has been found that the control of emesis can be improved by 15% to 20% with the addition of dexamethasone to 5-HT3 receptor antagonists.9 However, even with the wide availability of 5-HT3 antagonists, a third of all chemotherapy patients were not being controlled.2 Further research led to the development of NK1 receptor antagonists.
Presently, phenothiazines have little or no use in chemotherapy regimens associated with moderate-to-severe emesis.11 Nabilone, a cannabinoid, has shown superior activity over domperidone and prochlorperazine but lower efficacy than metoclopramide or chlorpromazine.12 Additionally, there seems to be no additional benefit when nabilone is added to a 5-HT3 receptor antagonist.12 Cannabinoids are therefore commonly used as second-line therapy.13
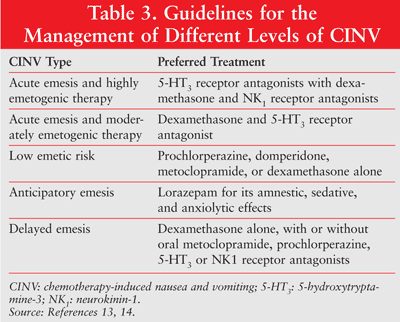
Various guidelines that have been published for the management of CINV are summarized in TABLE 3.13,14
5-HT3 Receptor Antagonists
The 5-HT3 receptor antagonists were a breakthrough in the management of CINV. Before the introduction of ondansetron, the first drug in this group, many patients would become so dehydrated that they would not be discharged until their fluid balance had been restored, which in some cases meant a few extra days in the hospital.15
5-HT3 receptors are the only ligand-gated ion channels in the serotonin receptor family, all others being G-protein coupled.16 5-HT3 receptors are found on the vagus nerve in the GI tract and in the vomiting center in the brain.15 By 2010, there were nine 5-HT3 receptor antagonists available, and all except alosetron are approved by the FDA for the management of NV.4 There are a further seven compounds in phase II trials and eight that have reached phase III trials.4
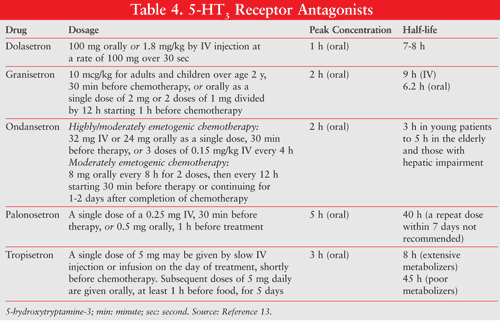
There are significant differences within the group of 5-HT3 antagonists, particularly in terms of onset and duration of action, as well as potential drug–drug interactions. Palonosetron, a second-generation drug in this class, has a longer half-life and therefore a higher rate of control of emesis as compared to ondansetron or dolasetron. However, at equipotent doses, granisetron, ondansetron, dolasetron, and tropisetron are effective and equally safe.13 The pharmacokinetics of each is highlighted in TABLE 4.
5-HT3 antagonists have been known to cause headache, a sensation of flushing or warmth, and constipation. A transient rise in liver enzymes has occasionally occurred. Serious side effects include leukopenia, thrombocytopenia, anemia, and QT prolongation. Rarely, chest pain, hypotension, tachycardia, and bradycardia have been reported. Dizziness and transient visual disturbances such as blurred vision have been observed during rapid IV administration. There have been rare reports of immediate hypersensitivity reactions, including anaphylaxis, taste changes, and alopecia.13
NK1 Receptor Antagonists
This is the newest group of antiemetics to be developed. The first drug of this group, aprepitant, was marketed in 2003 and is available as capsules.7 Fosaprepitant is a prodrug of aprepitant that is available in injectable form. The addition of aprepitant to a 5-HT3 receptor antagonist and corticosteroid regimen has further improved emetic control.9 Given that aprepitant is a CYP450 3A4 inhibitor, it interacts with other drugs that are metabolized by CYP3A4 enzymes, including chemotherapeutic agents such as ifosfamide.9 Since ifosfamide normally causes delayed NV, administering aprepitant the day after the dose of ifosfamide usually overcomes this interaction.9
Peak plasma concentrations following oral administration of aprepitant are seen about 4 hours after administration.13 Liver metabolism renders it inactive, and its terminal half-life is from 9 to 13 hours.13 The recommended dosage in combination with dexamethasone and a 5-HT3 receptor antagonist, for moderate to highly emetogenic chemotherapy, is as follows13:
• Day 1: 125 mg oral aprepitant 1 hour before chemotherapy followed by 12 mg oral dexamethasone and 32 mg IV ondansetron (for example) 30 minutes prior to chemotherapy. Alternatively, 115 mg of fosaprepitant can be substituted for 125 mg oral aprepitant 30 minutes prior to chemotherapy on day 1 only.
• Days 2 and 3: 80 mg oral aprepitant and 8 mg oral dexamethasone in the morning.
• Day 4: 8 mg dexamethasone in the morning.
Cannabinoids
Cannabinoids such as nabilone are usually used in cancer chemotherapy patients who have failed to adequately respond to conventional antiemetics.12 The usual starting dose for nabilone is 1 mg orally given twice daily starting 3 hours before chemotherapy, up to a maximum dose of 2 mg three times daily.13
Nabilone is commonly associated with drowsiness, confusion, euphoria, dizziness, hallucinations, psychosis, depression, headache, and tremors. Some patients may also complain of dry mouth, decreased appetite, and abdominal cramping.
Olanzapine
Most recently, the addition of olanzapine, an atypical antipsychotic, to a 5-HT3 receptor antagonist and dexamethasone regimen has shown highly promising results. However, more research is required to establish this drug as an effective agent in the treatment of CINV.9
Scopolamine
Scopolamine, also known as L-hyoscine, competitively inhibits muscarinic receptors. Transdermal scopolamine has demonstrated benefits in the management of severe drug-resistant NV in advanced cancer.16 A recent study showed that the use of scopolamine may have an antiemetic sparing effect in CINV.16 However, more case studies and trials need to be recorded for scopolamine to become integrated into the recommended regimen for the management of CINV.
Acupuncture
Acupuncture has been proven effective in management of CINV by the National Institutes of Health.17 It has been demonstrated that the insertion of the needles and stimulation of acupuncture points triggers the release of neurotransmitters, modulating the CINV. This is an option to present to patients who would like to try alternative remedies.
Management of CINV in Children
A recent Cochrane-based review suggests that the use of antiemetics in children is yet to be optimized and requires a great deal of research.18
Drug Interactions
Since the majority of patients undergoing chemotherapy suffer psychosocial distress in addition to CINV, there is a potential for drug interactions with drugs used to treat these co-existing conditions.19 It is important to take these interactions into consideration when selecting a suitable antiemetic or adjusting therapy.
5-HT3 receptor antagonists are quite effective, and palonosetron and granisetron in particular have had no interactions with many types of antidepressants and antipsychotics, including phenothiazines.19 Dolasetron, however, has to be used with caution because it has demonstrated serious drug–drug interactions with antidepressants and antipsychotics.19 Of particular importance are interactions with drugs that prolong the QT interval, including thioridazine and pimozide.
Since aprepitant is quite an effective antiemetic, it should be recommended when it is safe to use.19 It does interact, in many cases seriously, with antidepressants and antipsychotics, particularly nefazodone and pimozide.19 It is important to note that aprepitant is both a moderate inhibitor (within 14 days of therapy) and inducer of CYP3A4.13
Phenothiazines generally interact with most antidepressants and antipsychotics.19 Since many of these reactions are severe, this drug combination should be avoided.19 Lorazepam, which is used to treat anticipatory emesis, demonstrates a serious interaction with ziprasidone.13,19 Dexamethasone interacts with quetiapine and bupropion.13,19
Conclusion
This review has analyzed some of the options available in the amelioration of CINV. In order to improve adherence, it is pivotal for the pharmacist to counsel patients on the importance of taking their antiemetics at home. The pharmacist is in a unique position to collaborate with health care professionals and optimize care. Since the oncology health care team can be quite complex, consisting of surgeons, oncologists, and primary care providers, the pharmacist is in the best position to coordinate drug therapy as a consistently present member of the team.
REFERENCES
1. Chemotherapy-induced nausea and vomiting. Genentech USA, Inc. www.cancernausea.com/cinv/
2. Zanni GR. Help for chemotherapy-induced nausea and vomiting. Pharm Times. March 1, 2005. www.pharmacytimes.com/issue/
3. Trigg ME, Higa GM. Chemotherapy-induced nausea and vomiting: antiemetic trials that impacted clinical practice. J Oncol Pharm Pract. 2010;16:233-244.
4. Chong Y, Choo H. 5-HT3 antagonists under development. Expert Opin Investig Drugs. 2010;19:1309-1319.
5. McNatty D. Side effect solutions: helping patients avoid drug-induced nausea. Pharm Times. August 1, 2008. www.pharmacytimes.com/issue/
6. Tortorice PV, O’Connell MB. Management of chemotherapy-induced nausea and vomiting. Pharmacotherapy. 1990;10:129-145.
7. Herrstedt J, Dombernowsky P. Anti-emetic therapy in cancer chemotherapy: current status. Basic Clin Pharmacol Toxicol. 2007;101:143-150.
8. Warr DG. Chemotherapy- and cancer-related nausea and vomiting. Curr Oncol. 2008;15(suppl 1):S4-S9.
9. Frame DG. Best practice management of CINV in oncology patients: I. Physiology and treatment of CINV. Multiple neurotransmitters and receptors and the need for combination therapeutic approaches. J Support Oncol. 2010;8(suppl 1):5-9.
10. Goodman M. Risk factors and antiemetic management of chemotherapy-induced nausea and vomiting. Oncol Nurs Forum. 1997;24(suppl):20-32.
11. Triozzi PL, Laszlo J. Optimum management of nausea and vomiting in cancer chemotherapy. Drugs. 1987;34:136-149.
12. Davis MP. Oral nabilone capsules in the treatment of chemotherapy-induced nausea and vomiting and pain. Expert Opin Investig Drugs. 2008;17:85-95.
13. Sweetman SC, ed. Martindale: The Complete Drug Reference. 34th ed. London, UK: Pharmaceutical Press; 2005.
14. Kris MG, Hesketh PJ, Somerfield MR, et al. American Society of Clinical Oncology guidelines for antiemetics in oncology: update 2006. J Clin Oncol. 2006;24:2932-2947.
15. Bryan J. Ondansetron: landmark drugs. Pharm J. 2010;285:201-202.
16. LeGrand SB, Walsh D. Scopolamine for cancer-related nausea and vomiting. J Pain Symptom Manage. 2010;40:136-141.
17. Capodice JL. Acupuncture in the oncology setting: clinical trial update. Curr Treat Options Oncol. 2010;11:87-94.
18. Phillips RS, Gopaul S, Gibson F, et al. Antiemetic medication for prevention and treatment of chemotherapy induced nausea and vomiting in childhood. Cochrane Database Syst Rev. 2010;(9):CD007786.
19. Saylor MS, Smetana RF. Potential for drug-drug interactions in treating cancer-related nausea and distress. J Oncol Pharm Practice. October 1, 2010. http://opp.sagepub.com/
To comment on this article, contact rdavidson@uspharmacist.com.





