US Pharm. 2011;36(6)(Generic suppl):22-28.
Generic-drug products provide a cost-saving alternative to their branded counterparts. In 2010, generic products accounted for more than 78% of total prescriptions filled in the United States, compared to 57% in 2004.1,2 Although generics are used to fill the majority of prescriptions, the actual costs associated with them are significantly lower, accounting for less than 20% of the dollars spent on prescription purchases. Current estimates indicate that generic drug products sold through community pharmacies save consumers between $8 and $10 billion dollars yearly.3 While direct cost savings are a significant benefit of generic drugs, studies have also shown other advantages, including improvements in indirect costs such as therapy adherence and compliance, as well as increasing accessibility to health care.4
Although the affordability of generic drugs is well known, utilizing them appropriately and to their full potential may be challenging. Understanding their definition and modest history, as well as some key concepts related to generic substitution, challenges regarding narrow therapeutic index drugs, and nuances for patient counseling will help ensure that the use of generic medications is optimized.
Generic Drugs: Brief History, Approval Process, and Definition
Generic-drug products have been around since as early as the 1800s, when the pharmacy and medical communities in the U.S. were first established. The National Formulary published by the American Pharmaceutical Association (APhA) in the late 1800s was one of the first literary publications aimed at the prevention of brand-name counterfeits.5,6 The passage of the Federal Food and Drugs Act of 1906 and the Federal Food, Drug, and Cosmetic Act (FDCA) of 1938 helped to further ensure that the integrity and safety of medications were at the forefront.7,8 Subsequent legislation, including the Kefauver-Harris Drug Amendments of 1962, the Medicaid and Medicare amendments to the Social Security Act passed during the 1960s, and the Drug Price Competition and Patent Term Restoration Act of 1984 (frequently referred to as the Hatch-Waxman Act), was pivotal in further defining manufacturing procedures and ensuring healthy competition for prescription products with the availability of generics.5,7,9
The approval process for generic drugs differs from that for new chemical entities. Because safety and efficacy data have already been submitted and approved by the FDA for the pioneer product, manufacturers of generics must now prove that their product is bioequivalent through an Abbreviated New Drug Application (ANDA). According to the FDA, a generic drug is identical to a brand-name drug in safety, quality, performance characteristics, dosage form, strength, and route of administration, and has the same intended use as the innovator product. For the generic to be considered identical or bioequivalent, the FDA approval process ensures that the active ingredients are the same, although inactive ingredients may differ, and that the generic product withstands the rigorous testing required by the Center for Drug Evaluation and Research (CDER).9-11
Once a generic product is deemed bioequivalent, further evaluation is required for a drug to be therapeutically equivalent to, or substitutable for, a brand-name product. As a result, the generic drug is expected to have the same effect as the brand-name product, or no difference in effect when the generic is utilized in place of the branded drug. While this concept is true for generic products considered bioequivalent to their branded counterparts, bioequivalence among generic products may not always be the case, although their efficacy is not expected to differ significantly.12
Therapeutic Equivalence and Generic Substitution
Pharmacists in the dispensing role make frequent generic substitution decisions based on practical experience, as well as on substitution laws mandated by the federal government and the pharmacist’s state of practice. Most generic-drug products can be readily interchanged with the brand-name product; however, there are a few special considerations to take into account when making generic substitutions.
Federal Guidance: Perhaps the best reference for pharmacists to utilize when determining therapeutic equivalence for a generic product is the FDA’s CDER publication Approved Drug Products with Therapeutic Equivalence Evaluations, more commonly known as the Orange Book.11 The purpose of the Orange Book is to provide therapeutic equivalence evaluations for approved prescription-drug products with multiple sources or manufacturers.
The Orange Book assigns equivalence codes beginning with either an A or a B, which indicates that the FDA has evaluated a particular product as therapeutically equivalent to other pharmaceutically equivalent products. The first letter is paired with a second letter that provides additional information on the basis of the FDA’s evaluations. Codes beginning with an A indicate that the FDA considers the product to be therapeutically equivalent, with no known or suspected bioequivalence problems or with actual or potential bioequivalence problems that have been resolved with adequate in vivo and/or in vitro studies. Products designated with a B code are not considered therapeutically equivalent to other pharmaceutically equivalent products. Please refer to TABLE 1 for a listing of Orange Book equivalence codes and their definitions.11
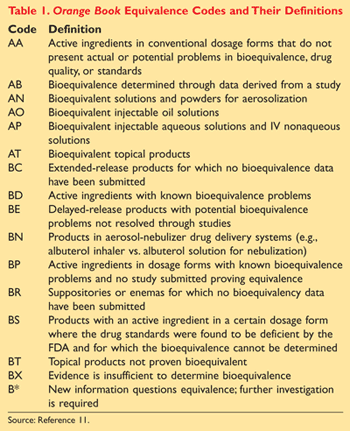
Of special importance are the limitations surrounding the use of the Orange Book. As the official name implies, the Orange Book only provides guidance for FDA-approved drug products that came to the market after the FDCA of 1938.11 Those drugs on the market prior to 1938 that are without a new drug application (NDA) were grandfathered in; therefore, they are not included in the Orange Book itself. Other special situations, including amino acid and protein hydrolysate injections, follitropin formulations, and levothyroxine products (described later), must be individually evaluated in the Orange Book before making generic substitution decisions. There are also a few equivalence codes containing numbers at the end, which indicate that two or more reference-listed drugs exist with the same active ingredient in the same strength but are not considered bioequivalent. Special attention should be given to the selection of generics for these products.11
State Guidance: In addition to federal law, all states in the U.S. have regulations regarding generic substitution. The degree to which states allow or restrict generic substitution varies greatly, as does their recognition of the guidelines set forth in the Orange Book. Additionally, states also differ on the leeway offered to their pharmacists to perform generic substitution, ranging from the allowance of generic substitution so long as drugs are pharmaceutically equivalent to prohibiting generic substitution without the authority of the prescriber. Some states also identify other drugs for which special circumstances apply.13
Substitution of NTI Drugs: Drugs with a narrow therapeutic index (NTI) have less than a twofold difference between the minimum toxic concentrations and the minimum effective concentrations in the blood.14 Some of the most notable examples of NTI drugs include digoxin, levothyroxine, and warfarin (TABLE 2). Other drug classes have also been identified for which concerns regarding generic substitution exist. While the FDA does not require any extraordinary conditions for the regulation of NTI generics, the appropriateness of generic substitution for these agents has come into question nonetheless.
Digoxin is not included in the Orange Book and is an example of a drug product that was grandfathered in because its availability predated the FDCA; therefore, generic digoxin products may be placed on the market without an approved ANDA. Because of issues surrounding bioavailability and the potential for unpredictable clinical response with generic digoxin products, generic substitution for brand-name digoxin (Lanoxin) should be performed with caution, even though the same response should be expected from seemingly therapeutically equivalent products.15
Levothyroxine sodium is another example of a drug in which generic substitution is awash in controversy. There are five different innovator products of levothyroxine, in addition to a number of generics.11 The equivalence coding for levothyroxine becomes challenging to interpret with the use of three characters among the different products, although the FDA accepts the bioequivalence and substitution of those products with like equivalence codes.16
The Orange Book currently lists three approved generic versions of warfarin sodium tablets.11 When generic warfarin first became available, questions arose as to the appropriateness of substituting it for brand-name Coumadin due to concerns regarding tablet content uniformity. A number of studies have since proven that no significant differences in the international normalized ratio (INR), dosage adjustments, or bleeding complications exist between products.15 Other factors, including drug interactions, dietary vitamin K intake, noncompliance, and other disease and lifestyle changes are more likely to affect anticoagulant response.
Concern has also arisen with a number of other drug classes for which generic substitution may be problematic. Generic products for antiepileptic drugs, antiarrythmics, transplant or antirejection medications, and antiasthmatics, particularly theophylline and albuterol metered-dose inhalers, have all come under scrutiny. Specific clinical circumstances should be closely evaluated before making decisions on generic substitution. While in a number of patients generic substitution is unlikely to result in any significant risk, patients should be carefully monitored, and, when appropriate, serum drug concentrations be closely followed. Many practitioners also argue that once a patient has been titrated and stabilized on a specific NTI product, switching between generic sources or back and forth between the brand and the generic product should be avoided.
A number of professional organizations, including the Academy of Managed Care Pharmacy (AMCP), oppose legislation that restricts the generic substitution of NTI drugs.17 Pharmacists should work in collaboration with a patient’s provider to make appropriate generic substitutions that are in the patient’s best interest where safety and cost-effectiveness are concerned. Review TABLE 2 for a list of NTI drugs or drug classes with potential generic substitution concerns.15,18

Patient Counseling Regarding Generics
Despite the availability of generic products and their associated cost savings, there are a number of reasons why patients may prefer brand-name products. Mistrust of generic medications based on perceived lack of efficacy and concerns over safety are common reasons. Additionally, patients commonly misunderstand the push to use generics as offering to save them money at the expense of their health.
Many of the misconceptions surrounding generic-medication use can be made clearer through education. Addressing patient concerns about medication costs can be an opportune time to suggest the utilization of generic products in order to save money. If concerns arise over safety and efficacy, pharmacists can counsel patients that for most products, the FDA requires rigorous testing to be done to ensure that the generic product meets the same safety requirements and is expected to have the same therapeutic response as the brand-name product. Patients can also be educated that paying a higher price for a brand-name product does not mean that they will experience a better outcome, especially if the cost of the medication is affecting adherence to a particular regimen.19
During the dispensing process, identifying medications with generic alternatives will also benefit the patient in terms of cost savings. In 2009, the top four drug classes by total number of prescriptions dispensed included the atypical antipsychotics, lipid-lowering agents, proton pump inhibitors, and antidepressants.2 While some of these classes have unique single-drug agents that are not readily interchangeable, most have a suitable generic product for the specific agent in question or a generic suitable enough to produce a desired class effect, which can lead to significant savings.
In addition to dispensing a drug product in the community, pharmacists in other practice settings can play a role in encouraging generic-medication use and identifying areas in a patient’s regimen in which cost savings might occur. In the hospital setting, medication reconciliation or discharge planning may both be areas in which issues regarding medication costs can be identified. Other opportunities include education at the time of prescribing, as well as medication therapy management (MTM) programs or other clinical pharmacy services.
Conclusion
Generic-drug products offer a number of advantages, including financial savings, increased medication accessibility, and improvement in the ability of patients to adhere to drug therapy regimens, particularly where multiple medications are involved. Understanding the logistical issues surrounding the generic-drug approval process, the regulations regarding generic substitution both nationally and statewide, and the practical considerations for NTI drugs, will all assist the pharmacist in utilizing generics appropriately. Pharmacists can also make a significant impact through proper education regarding generic-drug products and by suggesting their use when appropriate. Ultimately, these considerations will help ensure that the utilization of generic drugs is optimized and that their benefits, cost savings and otherwise, are realized.
REFERENCES
1. Gatyas G. IMS Institute reports U.S. spending on medicines grew 2.3 percent in 2010, to $307.4 billion. April 19, 2011. www.imshealth.com. Accessed April 27, 2011.
2. Gatyas G. IMS Health reports U.S. prescription sales grew 5.1 percent in 2009, to $300.3 billion. April 1, 2010. www.imshealth.com. Accessed April 9, 2011.
3. Generic Pharmaceutical Association. Facts at a glance. www.gphaonline.org. Accessed March 16, 2011.
4. Shrank WH, Hoang T, Ettner SL, et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med. 2006;166:332-337.
5. Ascione FJ, Kirking DM, Gaither CA, Welage LS. Historical overview of generic medication policy. J Am Pharm Assoc (Wash). 2001;41:567-577.
6. Higby GJ. The early history of USP. In: Anderson L, Higby GJ, eds. The Spirit of Voluntarism: A Legacy of Commitment and Contribution: The United States Pharmacopoeia 1820-1995. Rockville, MD: United States Pharmacopeial Convention; 1995:1-189.
7. Milestones in U.S. food and drug law history. About FDA. www.fda.gov/AboutFDA/WhatWeDo/
8. Meyer GF. History and regulatory issues of generic drugs. Transplant Proc. 1999;31(suppl 3A):10S-12S.
9. FDA Center for Drug Evaluation and Research. Office of Generic Drugs. What are generic drugs? www.fda.gov. Accessed March 16, 2011.
10. FDA Center for Drug Evaluation and Research. Office of Generic Drugs. Understanding generic drugs. www.fda.gov. Accessed March 16, 2011.
11. U.S. Department of Health and Human Services, FDA/CDER. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. 31st ed. 2011. www.fda.gov/cder/ob. Accessed March 16, 2011.
12. Generic drug variability. Pharmacist’s Letter/Prescriber’s Letter. 2008;24:240704.
13. Vivian JC. Generic-substitution laws. US Pharm. 2008;33(6)(Generic Drug Review suppl):30-34.
14. FDA Center for Drug Evaluation and Research. Office of Generic Drugs. Therapeutic equivalence of generic drugs—response to National Association of Boards of Pharmacy. www.fda.gov. Accessed March 16, 2011.
15. Henderson JD, Esham RH. Generic substitution: issues for problematic drugs. South Med J. 2001;94:16-21.
16. Bolton S. Bioequivalence studies for levothyroxine. AAPS J. 2005;7:E47-E53.
17. Academy of Managed Care Pharmacy. Interchange of narrow therapeutic index drugs. www.amcp.org. Accessed March 16, 2011.
18. FDA Center for Drug Evaluation and Research. Guidance for Industry. Bioavailability and bioequivalence studies for orally administered drug products—general considerations. www.fda.gov. Accessed March 16, 2011.
19. Smolen A. Medication therapy management: recommending generic drugs as cost-savings substitutes. US Pharm. 2010;35(6)(Generic Drug Review suppl):22-28.
To comment on this article, contact rdavidson@uspharmacist.com.






