US Pharm. 2011;36(7)(Oncology suppl):3-7.
ABSTRACT: Thyroid cancer is the most common cancer of the endocrine system. Over the past few decades, there has been a dramatic increase in the number of new patients diagnosed with this disease. Current treatment options include invasive surgery, radioiodine treatment, thyroid hormone suppressive therapy, external beam radiotherapy, and cytotoxic agents. Cytotoxic agents have been least effective in patients with metastatic thyroid cancer. Fortunately, several novel drugs are currently being evaluated in clinical trials to determine their efficacy against thyroid cancer.
Compared to other malignancies, thyroid cancer is relatively rare and accounts for only 1% of all neoplastic diseases.1 In 2009, it is estimated that 37,200 individuals were diagnosed with thyroid cancer, and approximately 1,590 patients died.2 These numbers are significantly lower than those of lung, breast, or prostate cancers. At the same time, it is also important to note that thyroid cancer is the most common cancer of the endocrine system, and the number of new cases has increased dramatically over the past few decades. In fact, the thyroid cancer rate in the United States doubled between 1972 and 2002.3 In women, thyroid carcinoma occurs two to three times more frequently than it does in men, with the disease being the sixth most prevalent cancer in women.2 Fortunately, patients with differentiated thyroid cancers (80% of all thyroid cancers are differentiated) have a fairly good prognosis and are cured by surgery and radioiodine treatment.2 This article will discuss the current treatment options for thyroid cancer as well as recent advances in new drug developments to treat this disease.
Risk Factors
Several risk factors have been identified that are associated with the development of thyroid cancer. A previous history of neck or head radiation treatment during infancy or childhood is the most important environmental factor linked to the development of thyroid cancer.4 In this age group, a malignant nodule may develop as early as 5 years following irradiation and may attain peak incidence within 30 years.4 For example, children and adolescents who were exposed to external radiation from the nuclear reactor disaster in Chernobyl developed papillary thyroid carcinomas in their later lives.5 Other risk factors include iodine deficiency, first-degree relatives of patients with differentiated thyroid carcinomas, presence of benign nodules or goiters, and hereditary conditions including familial adenomatous polypopsis (FAP), Cowden disease, and Carney syndrome.6-9 Although thyroid cancer is more common in women, the disease is often more fatal in men.6
Cellular Classification and Molecular Insights
Papillary Thyroid Carcinoma (PTC): This is the most common form and represents 80% of all thyroid cancers.10 Originating from the follicular cell of the thyroid, PTC is readily manageable and has a very good prognosis.10 These cancerous cells can uptake iodine readily and secrete thyroglobulin, which make detection and follow-up of the disease relatively simple.10 The key cellular pathways associated with PTCs include gene rearrangement of the RET proto-oncogene (rearranged during transfection), resulting in the formation of RET/PTC chimeric oncoproteins, as well as mutations of BRAF and RAS proto-oncogenes.11-13
Follicular Thyroid Carcinoma (FTC): This type accounts for 10% of thyroid cancers.10 In contrast to PTC, FTC has less lymph-node involvement, but distant metastases to the lungs and bone are often seen during presentation.10 Genetic abnormalities include translocation that results in fusion of DNA-binding segment of thyroid transcription factor PAX 8 gene, and coding sequence of peroxisome proliferator-activated receptor-g1 (PPAR-g1), a member of the nuclear hormone receptor family.14 Mutations in RAS proto-oncogenes are common in FTCs.13
Hürthle Cell Carcinoma (HCC): This is a modified form of FTC.10 Patients presenting with this disease have a greater probability of having distant metastases than patients with PTC and FTC.10 Presence of a large number of mitochondria in the cancerous cells is characteristic of HCC.15 Deletion mutations of mitochondrial DNA and mutations in somatic genes encoding mitochondrial proteins have been associated with HCC.15
Medullary Thyroid Carcinoma (MTC): Derived from calcitonin-secreting parafollicular C-cells of the thyroid, 80% of MTC cases are sporadic, and another 20% are hereditary in nature.16 The hereditary forms can occur with other tumors as multiple endocrine neoplasia type 2A and 2B (MEN 2A and MEN 2B) or isolated familial medullary thyroid carcinoma (FMTC).16 The hereditary forms of MTC result from a germ-line mutation in the RET proto-oncogenes.17
Anaplastic Thyroid Carcinoma (ATC): This is one of the most aggressive cancers and constitutes for 5% to 15% of all metastatic thyroid cancers.18 ATCs can form de novo or may result from dedifferentiation of PTC and FTC.18 The prognosis of ATC is poor, and death occurs within a few months of diagnosis. At the molecular level, mutations occur in p53, b-catenin, and PIK3CA genes.18 Mutations in RAS proto-oncogenes also occur in ATCs.18
Current Treatment Strategies
Near-total or total thyroidectomy followed by thyroid remnant ablation with radioiodine therapy has been the major treatment option for differentiated thyroid carcinomas (e.g., PTCs and FTCs).9,10 For thyroid remnant ablation, elevation of endogenous thyroid-stimulating hormone (TSH) level is necessary for adequate uptake of radioiodine by thyroid carcinoma cells. This is readily achieved by either withdrawal of synthetic thyroid hormone medication or by administering recombinant TSH (Thyrogen) along with thyroid hormone supplementation.19 Once taken up by the thyroid cells, I131 undergoes radioactive decay, emitting high-energy electron particles and causing the death of neoplastic cells.9,10 For MTC, total thyroidectomy along with dissection of the central neck compartment is indicated. In addition, patients suffering from MTC may be treated with adjuvant radiotherapy.9,10 Although treatment for ATC is predominantly supportive, in certain cases surgery in conjunction with external beam radiation might be beneficial.9,10 Cytotoxic agents have limited efficacy in advanced, metastatic thyroid carcinomas.
Doxorubicin, an anthracycline antibiotic, is the only chemotherapeutic agent approved for the treatment of metastatic thyroid carcinoma. Its antitumor effects include inhibition of RNA and DNA synthesis, generation of highly reactive, cytotoxic free-radicals, and alteration of membrane fluidity and ion transport of cancer cells.20 The drug provides partial and temporary relief in patients with advanced thyroid carcinoma.21 Patients with pulmonary metastases responded better to doxorubicin treatment than those with bone or nodal metastases.9 The recommended dose of doxorubicin (monotherapy) is 60 to 75 mg/m2 IV bolus every 3 weeks. Adverse effects reported from clinical trials include irreversible cardiomyopathy, cardiac arrhythmias, nausea, vomiting, infections, granulocytopenias, infertility, and alopecia.9
Other than paclitaxel, which provides some benefit in ATC,22 cytotoxic agents when used alone or in combination are essentially ineffective against thyroid carcinoma.23
An important breakthrough in thyroid cancer treatment is the recent introduction of an agent for the treatment of MTC. Vandetanib, an orally active kinase inhibitor, has been approved this year for the treatment of MTC in patients with unresectable locally advanced or metastatic disease.24 Vandetanib inhibits kinases associated with VEGF, RET, and epidermal growth factor receptor (EGFR) pathways. In a double-blind, placebo-controlled, randomized trial, patients with unresectable locally advanced or metastatic MTC (n = 231), showed significant improvement in progression-free survival with vandetanib 300 mg daily when compared to placebo control (n = 100). The approved dose for vandetanib is 300 mg once daily with or without food. The agent is associated with several adverse effects including a boxed warning of QT prolongation, torsades de pointes, and sudden death. Vandetanib should not be used in patients with hypocalcemia, hypokalemia, hypomagnesemia, or prolonged QT syndrome. QT interval should be monitored by ECG recordings at baseline and at different time intervals during vandetanib treatment. Other serious adverse effects reported with vandetanib treatment include rash (i.e., Stevens-Johnson syndrome) and interstitial lung disease.24
Investigational Agents
Several compounds are currently undergoing clinical trials for anticancer effects in the thyroid. The findings of these trials and the mechanism of action of these investigational agents are discussed in the following section (TABLE 1).
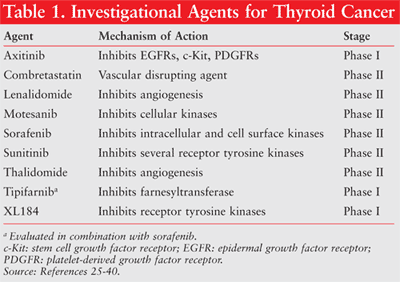
Sorafenib is an orally active multiple kinase inhibitor that is indicated for the treatment of unresectable hepatocellular carcinoma and advanced renal cell carcinoma.25 It inhibits several intracellular and cell surface kinases including platelet-derived growth factor receptors (PDGFRs), vascular endothelial growth factor receptors (VEGFRs), BRAF, and RET. Three phase II studies have been conducted to evaluate sorafenib’s efficacy in advanced thyroid cancers. In the first study, 30 patients with metastatic, radioiodine-resistant thyroid carcinomas of all histological subtypes were included.26 Sorafenib administered at a dose of 400 mg twice daily for at least 16 weeks resulted in seven patients attaining a partial response and 16 others with stable disease for at least 14 weeks. Significant adverse effects observed were hand-foot syndrome, rash, diarrhea, and hypertension. A patient died of liver failure that was likely related to the treatment. Seventeen out of 19 patients for whom serial thyroglobulin were measurable showed a mean decrease of 70%.26
In a separate phase II trial with sorafenib, 58 patients with thyroid cancer were recruited for a 10-month period.27 Of 41 PTC patients, six had a partial response and 23 had stable disease lasting at least 6 months. In 14 out of 18 thyroglobulin-assessable PTC patients, thyroglobulin levels were decreased by more than 25%. BRAF mutations were detected in 17 patients with PTC. Tumor biopsies from sorafenib-treated PTC patients detected a reduction in phosphorylation of VEGF as well as a decrease in VEGF expression, suggesting inhibition of angiogenesis.27
In a single-center, prospective phase II trial, 31 patients with progressive metastatic or locally advanced radioiodine refractory differentiated thyroid carcinoma (DTC) were treated with sorafenib 400 mg PO twice daily for 26 weeks.28 An endpoint of the study was the reinduction of radioiodine uptake after 26 weeks of drug treatment. Although eight patients achieved a partial response and 11 others had stable disease, sorafenib was found to be less effective in patients with bone metastases. There was no reinduction of radioiodine uptake at metastatic sites.28
Tipifarnib, a farnesyltransferase inhibitor, when combined with sorafenib in a phase I trial produced promising results in patients with MTC.29 Six out of eight MTC patients either had stable disease or a partial remission lasting over 2 years. In these patients, RET mutation was detected. The dose-limiting toxicity of this dual drug therapy was rash.29
Similar to sorafenib, sunitinib is an orally active agent that blocks several receptor tyrosine kinases including VEGFRs, RET, KIT, and PDGFR. This agent is approved by the FDA to treat gastrointestinal stromal tumor (GIST) and advanced renal cell carcinoma.30 In an open-label, phase II study, patients with progressive DTC or MTC were treated with 50 mg sunitinib once daily on a 4-week-on/2-week-off schedule per cycle.31 Findings from this study were partial response in 13% and stable disease in 68% of patients with DTC. Stable disease was achieved in 83% of MTC patients. Severe adverse effects included neutropenia, thrombocytopenia, hypertension, fatigue, palmar plantar erythrodysesthesia, and GI events.31 In a second phase II trial, partial response or stable disease lasting more than 12 weeks was reported in two of 12 DTC patients and three of eight MTC patients.32 Both sorafenib and sunitinib can be used selectively in thyroid cancer patients who do not otherwise qualify for clinical trials.
Axitinib is an orally active agent that inhibits vascular EGFR subtypes 1, 2, and 3 at subnanomolar concentrations. It also inhibits stem cell growth factor receptor (c-Kit) and PDGFR-b at higher concentrations. In a multicenter phase I study, patients with metastatic thyroid carcinomas were treated with axitinib at a dose of 5 mg twice daily.33 Partial response rate was 30% (which included patients with DTC, MTC, and ATC), and 38% of patients presented stable state lasting at least 4 months. Median progression-free survival lasted for 18 months. Hypertension occurred in 12% of patients.33
Motesanib (AMG-706) acts in a similar fashion as axinitib. It also inhibits RET at nanomolar concentration in vitro. An international, multicenter phase II trial of motesanib recruited 93 patients with radioiodine-refractory DTC.34 Patients included in the study had those with PTCs (61%) as well as FTCs (34%). Motesanib was administered orally at a dose of 125 mg daily. Fourteen percent of patients achieved partial response, 67% had stable disease, and 35% of those remained with stable disease for at least 6 months. The median progression-free survival period was 10 months. Of the motesanib-treated patients in whom thyroglobulin was analyzed, 81% had a marked decrease in thyroglobulin levels when compared with baseline values. The most common adverse effects observed in this trial were diarrhea, hypertension, weight loss, and fatigue.34
Another multicenter study evaluated motesanib’s efficacy in 91 patients with advanced MTC.35 Only two patients had a definitive partial response, which lasted between 5 to 8 months. Seven patients did not respond to the therapy, and their disease continued to progress. Stable disease was achieved in 81% patients, and it was maintained by 48% of them for 24 weeks or longer. The median progression-free survival period was 12 months. Serum calcitonin and carcinoembryonic antigen levels were decreased in 83 and 63 patients, respectively. An unexpected adverse effect of motenasib therapy was a 30% increase in the mean dosages of levothyroxine required to maintain TSH suppression/euthyroidism in both DTC and MTC cohorts.35
XL184 is an orally active tyrosine kinase inhibitor that blocks VEGFRs, RET, c-Kit, and c-Met. The c-Met gene encodes for hepatocyte growth factor receptors, which have increased expression in PTC and MTC.36 A phase I, multicenter, dose-finding study examined patients with different metastatic cancers, with an expansion cohort of MTC.37 Twelve patients (55%) with MTC attained partial response and taken together 84% of patients with MTC achieved partial response or stable disease that lasted more than 3 months. The most common adverse effects included diarrhea, nausea, anorexia, fatigue, mucositis, hypertension, and elevation of serum enzymes including ALT, AST, and lipase. It appears that in addition to decreasing the blood supply to tumors, the suppression of the compensatory mechanism (i.e., c-Met) is beneficial in MTC.37
Thalidomide’s antiangiogenic properties were evaluated in an open-label, phase II trial in patients with progressive DTC and MTC.38 With a starting dose of 200 mg of thalidomide daily, the daily dose was gradually increased to 800 mg or to the maximum tolerated dose over a 6-week period. Eighteen percent of the evaluable subjects attained a partial response and another 28% had stable disease. The median survival period was almost doubled in responders compared to nonresponders. Fifty percent of the patients discontinued thalidomide treatment because of adverse effects. Side effects most frequently encountered were fatigue, somnolence, constipation, peripheral neuropathy, dizziness, and infection.38
In a phase II trial, lenalidomide, a thalidomide analog with a different side effect profile, was administered orally at a daily dose of 25 mg to patients with radioiodine-resistant DTC.39 Preliminary findings among 18 evaluable patients suggest that 22% had a partial response and 44% achieved stable disease.39
Combretastatin (CA4P) is a tubulin-binding vascular disrupting agent that inhibits blood flow through blood vessels. A phase II clinical trial evaluated the anticancer properties of combretastatin in patients with metastatic ATC.40 Combretastatin was administered as a 10-minute IV infusion at a dose of 45 mg/m2 on days 1, 8, and 15 of every 28-day cycle. Findings indicate that 28% of patients were progression free for over 3 months and median survival time was approximately 5 months.40
Conclusion
Cytotoxic agents, when used either alone or in combination, have not provided much benefit in thyroid cancer. These drugs can also cause serious adverse effects by nonselectively destroying the healthy cells in the body. Novel therapeutic agents with well-defined molecular targets may be a better alternative in treating this disease.
REFERENCES
1. Franceschi S, Boyle P, Maisonneuve P, et al. The epidemiology of thyroid carcinoma. Crit Rev Oncog. 1993;4:25-52.
2. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249.
3. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164-2167.
4. Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiation Res. 1995;141:259-277.
5. Tronko MD, Howe GR, Bogdanova TI, et al. A cohort study of thyroid cancer and other thyroid diseases after the Chernobyl accident: thyroid cancer in Ukraine detected during first screening. J Natl Cancer Inst. 2006;98:897-903.
6. Wartofsky L. Increasing world incidence of thyroid cancer: increased detection or higher radiation exposure? Hormones (Athens). 2010;9:103-108.
7. Handkiewcz-Junak D, Banasik T, Kolosza Z, et al. Risk of malignant tumors in first-degree relatives of patients with differentiated thyroid cancer—a hospital based study. Neoplasma. 2006;53:67-72.
8. Giusti F, Falchetti A, Franceschelli F, et al. Thyroid cancer: current molecular perspectives. J Oncol. 2010;2010:351679.
9. Sherman SI. Thyroid carcinoma. Lancet. 2003;361:501-511.
10. Gasent Blesa JM, Grande Pulido E, Provencio Pulla M, et al. Old and new insights in the treatment of thyroid carcinoma. J Thyroid Res. 2010;2010:27946.
11. Bongarzone I, Vigneri P, Mariani L, et al. RET/NTRK1 rearrangements in thyroid gland tumors of the papillary carcinoma family: correlation with clinicopathological features. Clin Cancer Res. 1998;4:223-228.
12. Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625-627.
13. Karga H, Lee JK, Vickery AL Jr, et al. Ras oncogene mutations in benign and malignant thyroid neoplasms. J Clin Endocrinol Metab. 1991;73:832-836.
14. Kroll TG, Sarraf P, Pecciarini L, et al. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma. Science. 2000;289:1357-1360.
15. Máximo V, Soares P, Lima J, et al. Mitochondrial DNA somatic mutations (point mutations and large deletions) and mitochondrial DNA variants in human thyroid pathology: a study with emphasis on Hurthle cell tumors. Am J Pathol. 2002;160:1857-1865.
16. Chen H, Roberts JR, Ball DW, et al. Effective long-term palliation of symptomatic, incurable metastatic medullary thyroid cancer by operative resection. Ann Surg. 1998;227:887-895.
17. Santoro M, Carlomagno F, Romano A, et al. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science. 1995;267:381-383.
18. Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16:17-44.
19. Pacini F, Molinaro E, Castagna MG, et al. Ablation of thyroid residues with 30 mCi 131I: a comparison in thyroid cancer patients prepared with recombinant human TSH or thyroid hormone withdrawal. J Clin Endocrinol Metab. 2002;87:4063-4068.
20. Chu E, Sartorelli AC. Katzung’s Basic and Clinical Pharmacology. 11th ed. New York, NY: McGraw Hill Lange; 2009.
21. Haugen BR. Management of the patient with progressive radioiodine non-responsive disease. Semin Surg Oncol. 1999;16:34-41.
22. Ain KB, Egorin MJ, DeSimone PA. Treatment of anaplastic thyroid carcinoma with paclitaxel: phase 2 trial using ninety-six-hour infusion. Collaborative Anaplastic Thyroid Cancer Health Intervention Trials (CATCHIT) Group. Thyroid. 2000;10:587-594.
23. Droz JP, Schlumberger M, Rougier P, et al. Chemotherapy in metastatic nonanaplastic thyroid cancer: experience at the Institut Gustave-Roussy. Tumori. 1990;76:480-483.
24. Vandetanib (vandetanib) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; April 2011.
25. Nexavar (sorafenib) package insert. Wayne, NJ: Bayer HealthCare Pharmaceuticals Inc; 2005.
26. Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714-4719.
27. Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675-1684.
28. Hoftijzer H, Heemstra KA, Morreau H, et al. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2009;161:923-931.
29. Hong DS, Sebti SM, Newman RA, et al. Phase I trial of a combination of the multikinase inhibitor sorafenib and the farnesyltransferase inhibitor tipifarnib in advanced malignancies. Clin Cancer Res. 2009;15:7061-7068.
30. Sutent (sunitinib) package insert. New York, NY: Pfizer, Inc; 2006.
31. Cohen EEW, Needles BM, Cullen KJ, et al. Phase 2 study of sunitinib in refractory thyroid cancer. J Clin Oncol. 2008;26(suppl):A6025.
32. Ravaud A, de la Fouchardière C, Courbon F, et al. Sunitinib in patients with refractory advanced thyroid cancer: the THYSU phase II trial. J Clin Oncol. 2008;26(suppl):A6058.
33. Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708-4713.
34. Sherman SI, Wirth LJ, Droz JP, et al; Motesanib Thyroid Cancer Study Group. Motesanib diphosphate in progressive differentiated thyroid cancer. N Eng J Med. 2008;359:31-42.
35. Schlumberger M, Elisei R, Bastholt L, et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol. 2009;27:3794-3801.
36. Papotti M, Olivero M, Volante M, et al. Expression of hepatocyte growth factor (HGF) and its receptor (MET) in medullary carcinoma of the thyroid. Endocr Pathol. 2000;11:19-30.
37. Kurzrock R, Sherman S, Hong D, et al. A phase I study of XL184, a MET, VEGFR2, and RET kinase inhibitor, administered orally to patients (pts) with advanced malignancies, including a subgroup of pts with medullary thyroid cancer (MTC). Proceedings of the 20th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics; Geneva, Switzerland. 2000;119:A379.
38. Ain KB, Lee C, Williams KD. Phase II trial of thalidomide for therapy of radioiodine-unresponsive and rapidly progressive thyroid carcinomas. Thyroid. 2007;17:663-670.
39. Ain KB, Lee C, Holbrook K, et al. Phase II study of lenalidomide in distantly metastatic, rapidly progressive, and radioiodine-unresponsive thyroid carcinomas: preliminary results. J Clin Oncol. 2008;26(suppl):A6027.
40. Cooney M, Savvides P, Agarwala S, et al. Phase II study of combretastatin A4 phosphate (CA4P) in patients with advanced anaplastic thyroid carcinoma (ATC). J Clin Oncol. 2006;24(suppl):A5580.
To comment on this article, contact rdavidson@uspharmacist.com.





