ABSTRACT: Cytotoxic agents remain the major chemotherapeutic treatment modality for most types of cancer. However, chemotherapeutic agents are nonselective, damaging healthy cells and sometimes resulting in treatment failure. Novel drug delivery systems have been developed to address these problems. Two types of drug delivery systems—liposomal and albumin nanoparticle drugs—have caused notable improvement in the therapeutic index of anticancer agents; however, the lack of success in improving overall survival makes it imperative to further develop and refine drug delivery systems.
Cancer is a complex disease that is caused by the accumulation of genetic and epigenetic changes that allow cells to escape normal cellular controls. In 2011, Hanahan and Weinberg proposed hallmarks of cancer that enable cell growth and metastasis.1 The proposed hallmarks, which provide a framework for understanding the biology of cancer, are: (1) sustainment of proliferative signaling; (2) evasion of growth suppressors; (3) activation of invasion and metastasis; (4) enabling of replicative immortality; (5) inducement of angiogenesis; (6) resistance to cell death; (7) avoidance of immune destruction; and (8) deregulation of cellular energetics.1 Some drugs that interfere with certain hallmarks of cancer have been approved; others are in development or are being investigated in clinical trials.
Cytotoxic agents remain the major chemotherapeutic treatment modality for most types of cancer. Chemotherapeutics work primarily by interfering with DNA synthesis and mitosis, leading to the death of rapidly growing and dividing cancer cells. However, the agents are nonselective and can damage normal cells, frequently resulting in treatment failure. Consequently, novel drug delivery systems—such as nanoparticle-based therapeutics—have been extensively investigated in an effort to improve the therapeutic effectiveness and safety profile of traditional chemotherapeutic agents. These systems represent a multibillion-dollar-a-year industry. Several novel delivery systems have been approved for cancer therapy and are used in the clinic. This article will discuss liposomal and albumin nanoparticle–based drug delivery systems incorporating traditional chemotherapeutic agents for cancer therapy and will review their pharmacokinetic and pharmacologic properties, therapeutic efficacy, and safety profile.
Cancer Therapy
Surgery, radiation therapy, and chemotherapy are the principal treatment strategies for cancer. Of these modalities, surgery is most effective at an early stage of disease progression. Radiation therapy can damage healthy cells, organs, and tissues. Although chemotherapy has reduced morbidity and mortality, virtually all chemotherapeutic agents damage healthy cells, especially rapidly dividing and growing cells.2,3
As noted, chemotherapeutic agents can damage the DNA of cancer cells and inhibit DNA synthesis, thus inhibiting mitosis and stopping replication (i.e., preventing the original cancer cell from splitting into two new cells). Most chemotherapeutic agents are low-molecular-weight (LMW) compounds. They have a high volume of distribution and travel throughout the body. LMW chemotherapeutic agents are removed rapidly by renal excretion, and they are not protected from enzymatic degradation.
Drug resistance, a major problem with chemotherapy, is a phenomenon wherein cancer cells that initially were suppressed by an anticancer drug develop resistance to the drug.4 This is caused primarily by reduced drug uptake and increased drug efflux. In addition, chemotherapeutic agents have a narrow therapeutic index. Each drug has a distinct mechanism of action that may have different effects on varied types of cancer cells. For these reasons, cancer chemotherapy may consist of several drugs used in combination. Consequently, novel therapeutic agents and drug delivery systems for cancer therapy are needed to reduce generalized toxicity and to increase therapeutic effect.5
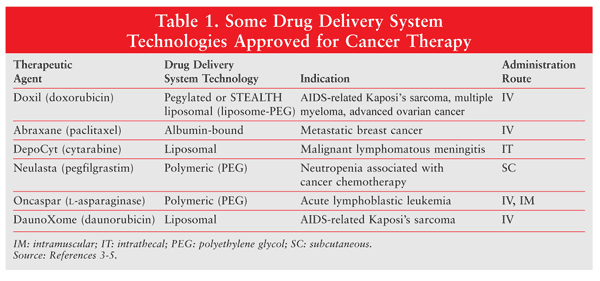
Numerous drug delivery systems (e.g., nanoparticles) and drug targeting systems (e.g., monoclonal antibodies) have been approved or are in development. These systems have emerged in an effort to overcome the lack of specificity of conventional chemotherapeutic agents. They may be designed to improve the pharmacologic and therapeutic properties of traditional chemotherapy agents. Nanoparticles, whose size ranges from a few nm to several hundred nm depending upon the intended use, have great potential for drug delivery and targeting of the tumor. Nanoparticle-based drug delivery systems include liposomes, micelles, and albumin microparticles.6,7 TABLE 1 lists some drug delivery system technologies or platforms that have been approved for cancer therapy.3-5 Of these systems, liposomal drug delivery is the most successful and is used to treat breast and ovarian cancers and Kaposi’s sarcoma.
Liposomal Drugs
Liposomes constitute one of the most widely investigated drug delivery systems for the treatment of cancer. Several liposomal delivery systems have been approved for cancer therapy (TABLE 1). Liposomes intended for cancer treatment are about 100 nm in diameter, as this size allows the drug carrier to extravasate from circulation through the leaky vasculature supporting the tumor.8 A liposome has many advantages over the corresponding nonliposomal drug: It has favorable pharmacokinetic properties, protects the therapeutic agent from enzymatic degradation, increases antitumor efficacy, and reduces generalized toxicity.8-10 Encapsulation of the drug helps minimize the unintended side effects of commonly used chemotherapeutics, such as the cardiotoxicity that generally results from the use of anthracyclines.
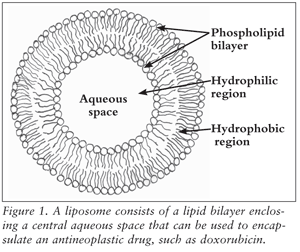
A schematic representation of a liposome is shown in FIGURE 1. Liposomes contain an internal aqueous core surrounded by a phospholipid bilayer. The internal aqueous core, which is used for drug encapsulation, is suited for the delivery of hydrophilic drugs, and the phospholipid bilayer allows for the delivery of hydrophobic drugs.10,11
Anthracyclines represent a class of potent chemotherapeutic agents that are constrained by systemic toxicities.12 Myocet and Doxil, both of which contain doxorubicin, were among the first liposomal products to be approved by the regulatory authorities.5,8 Doxorubicin, an anthracycline, is commonly used in the treatment of a wide range of cancers. It binds to the DNA of cancer cells, thus inhibiting nucleic acid synthesis; it also interferes with topoisomerase II activity and induces mutations and aberrations that can lead to the cell’s death. The difference between Myocet and Doxil is that Doxil is formulated with a polymer coating. Whereas in Myocet doxorubicin is entrapped in uncoated liposomes, in the Doxil system the surface of the liposome is grafted with polyethylene glycol (PEG).13,14 The unique PEG coating allows the STEALTH (also known as pegylated or sterically stabilized) liposome to evade recognition by the mononuclear phagocyte system.5,8,14,15 The pegylated liposomal particles are approximately 100 nm in size.
To date, Doxil has achieved the most prolonged circulation of the liposomal drugs, with a terminal half-life of 55 hours in humans.16 Doxorubicin hydrochloride is encapsulated in long-circulating or STEALTH liposomes for IV administration. Doxil is indicated for ovarian cancer after failure of platinum-based chemotherapy, for multiple myeloma in combination with bortezomib, and for AIDS-related Kaposi’s sarcoma. In the case of ovarian cancer, the dosing is 50 mg/m2 IV over 1 hour every 4 weeks for a minimum of four courses (TABLE 2).14,17,18
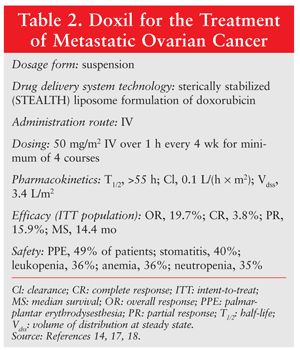
The pivotal trial that led to the approval of Doxil involved 474 patients with epithelial ovarian cancer that recurred after or failed to respond to first-line platinum-based chemotherapy.17,18 The open-label trial randomized 239 patients to Doxil 50 mg/m2 every 4 weeks and 235 patients to topotecan 1.5 mg/m2/day for 5 consecutive days every 3 weeks. The overall response rates for Doxil and topotecan were 19.7% and 17.0%, respectively. Overall, median survival was 14.4 months in the Doxil group and 13.7 months in the topotecan group, yielding an 18% reduction in risk of death for the intent-to-treat population. The most common adverse effects associated with Doxil were palmar-plantar erythrodysesthesia (49% of patients), stomatitis (40%), leukopenia (36%), anemia (36%), and neutropenia (35%). Overall, the toxicity attributed to Doxil was mild to moderate in severity.
Albumin Nanoparticle Drugs
Abraxane (also known as nab-paclitaxel), a protein-bound form of paclitaxel, is the first approved albumin nanoparticle drug.5,19 Paclitaxel, which is fundamental in the treatment of breast cancer, is an antimicrotubule agent. It stabilizes microtubule dynamics by preventing depolymerization, with consequent arrest of the cell cycle followed by apoptosis. As paclitaxel is highly hydrophobic and has poor water solubility, standard paclitaxel is formulated with a lipid-based solvent (ethanol and Cremophor EL [polyoxyethylated castor oil]) as a vehicle to improve drug delivery.20 This solvent system is associated with serious and dose-limiting toxicities.
Composed of albumin bound to paclitaxel, Abraxane is used for the treatment of metastatic breast cancer.19 Albumin, a plasma protein with a molecular weight of 66 kDa, has been extensively studied as a drug carrier. It is nontoxic and is well tolerated by the immune system.21 Albumin has a long half-life in circulating plasma, which makes it attractive as a drug carrier. This protein can be derived from human plasma and blood products.21
The major trial influencing FDA approval of Abraxane was an international, randomized, open-label, phase III study comparing the efficacy and safety of Abraxane versus standard paclitaxel (Taxol) in 454 patients with metastatic breast cancer.22 The Abraxane regimen was 260 mg/m2 given by 30-minute infusion without premedication every 3 weeks, and the dosing for standard paclitaxel was 175 mg/m² given by 3-hour infusion with routine steroid pretreatment every 3 weeks.22
The primary objective of the trial was to determine the noninferiority or superiority of Abraxane versus standard paclitaxel based on best confirmed response after six cycles of treatment. TABLE 3 gives a comparison of Abraxane and standard paclitaxel.19,20,22,23 Abraxane resulted in a significantly higher overall response rate compared with standard paclitaxel (33% vs. 19%) and a significantly longer time to tumor progression (23 wk vs. 16.9 wk). It likewise effected a significant improvement in response rate.22
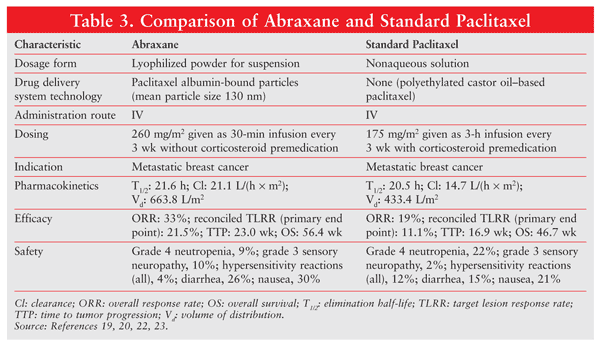
Use of the albumin-paclitaxel conjugate also resulted in increased clearance (43% larger) and volume of distribution (53% higher) of the active drug.19,23 Abraxane has an additional advantage over standard paclitaxel in that it lacks the hypersensitivity-inducing Cremophor EL solvent. The incidence of grade 4 neutropenia was significantly lower in Abraxane patients despite a 49% higher drug dose; however, grade 3 sensory neuropathy was more common in this group. Overall, Abraxane showed greater clinical efficacy compared with standard paclitaxel and had a favorable safety profile.23
Formulated as a lyophilized powder for injectable suspension, Abraxane is used for the treatment of metastatic breast cancer after failure of combination chemotherapy or relapse within 6 months of adjuvant chemotherapy. Prior therapy should include an anthracycline, unless this was clinically contraindicated.19,23
Conclusion
Current treatment strategies for cancer suffer from severe limitations, including nonspecific biodistribution of the chemotherapeutic agent, dose-limiting toxicity, and emerging drug resistance. As a result, novel cancer therapies such as liposomal drug delivery systems have rapidly evolved.
Since the approval of Doxil liposomal drug delivery system, novel and targeted drug delivery systems have become additions to the anticancer drug arsenal. Several delivery systems incorporating anticancer agents are currently on the market. Drug delivery systems, particularly liposomes, have been shown to improve the pharmacokinetic and pharmacologic parameters of conventional chemotherapeutics. In addition, response rates for some cancer types have shown improvement. Drug delivery systems also decrease the toxicity that often results from the use of chemotherapeutic agents.
Although drug delivery systems result in a substantial improvement in the therapeutic index of anticancer agents, overall survival has not improved significantly. As a result, further development and refinement of drug delivery systems are essential for improving therapeutic outcomes.
References
1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674.
2. Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol. 2008;26:57-64.
3. Moses MA, Brem H, Langer R. Advancing the field of drug delivery: taking aim at cancer. Cancer Cell. 2003;4:337-341.
4. Shapira A, Livney YD, Broxterman HJ, Assaraf YG. Nanomedicine for targeted cancer therapy: towards the overcoming of drug resistance. Drug Resist Updat. 2011;14:150-163.
5. Zhang L, Gu FX, Chan JM, et al. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761-769.
6. Koo OM, Rubinstein I, Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine. 2005;1:193-212.
7. Cho K, Wang X, Nie S, et al. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310-1316.
8. Brown DM, ed. Drug Delivery Systems in Cancer Therapy. Totowa, NJ: Humana Press; 2004.
9. Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4:95-99.
10. New RR, ed. Liposomes: A Practical Approach. New York, NY: Oxford University Press; 1990.
11. Khan DR, Rezler EM, Lauer-Fields J, Fields GB. Effects of drug hydrophobicity on liposomal stability. Chem Biol Drug Res. 2008;71:3-7.
12. Hortobágyi GN. Anthracyclines in the treatment of cancer. An overview. Drugs. 1997;54(suppl 4):1-7.
13. Myocet (doxorubicin HCl [liposome] for injection) package insert. Princeton, NJ: Elan Pharmaceuticals; 2000.
14. Doxil (doxorubicin HCl liposome injection) package insert. Raritan, NJ: Ortho Biotech Products LP; 2001.
15. Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs. 1997;54(suppl 4):15-21.
16. Gabizon A, Catane R, Uziely B, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene glycol coated liposomes. Cancer Res. 1994;54:987-992.
17. Gordon AN, Fleagle JT, Guthrie D, et al. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312-3122.
18. Gordon AN, Tonda M, Sun S, et al. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol. 2004;95:1-8.
19. Abraxane (paclitaxel protein-bound particles for injectable suspension) package insert. Bridgewater, NJ: Abraxis BioScience, LLC; March 2010.
20. Taxol (paclitaxel) package insert. Princeton, NJ: Bristol-Myers Squibb; 2003.
21. Miele E, Spinelli GP, Miele E, et al. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int J Nanomedicine. 2009;4:99-105.
22. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794-7803.
23. Sparreboom A, Scripture CD, Trieu V, et al. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol). Clin Cancer Res. 2005;11:4136-4143.
To comment on this article, contact rdavidson@uspharmacist.com.





